Insights into the Cellular Localization and Functional Properties of TSPYL5 Protein
- PMID: 38203210
- PMCID: PMC10779080
- DOI: 10.3390/ijms25010039
Insights into the Cellular Localization and Functional Properties of TSPYL5 Protein
Abstract
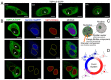
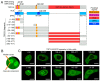
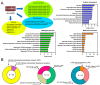
In recent years, the role of liquid-liquid phase separation (LLPS) and intrinsically disordered proteins (IDPs) in cellular molecular processes has received increasing attention from researchers. One such intrinsically disordered protein is TSPYL5, considered both as a marker and a potential therapeutic target for various oncological diseases. However, the role of TSPYL5 in intracellular processes remains unknown, and there is no clarity even in its intracellular localization. In this study, we characterized the intracellular localization and exchange dynamics with intracellular contents of TSPYL5 and its parts, utilizing TSPYL5 fusion proteins with EGFP. Our findings reveal that TSPYL5 can be localized in both the cytoplasm and nucleoplasm, including the nucleolus. The nuclear (nucleolar) localization of TSPYL5 is mediated by the nuclear/nucleolar localization sequences (NLS/NoLS) identified in the N-terminal intrinsically disordered region (4-27 aa), while its cytoplasmic localization is regulated by the ordered NAP-like domain (198-382 aa). Furthermore, our results underscore the significant role of the TSPYL5 N-terminal disordered region (1-198 aa) in the exchange dynamics with the nucleoplasm and its potential ability for phase separation. Bioinformatics analysis of the TSPYL5 interactome indicates its potential function as a histone and ribosomal protein chaperone. Taken together, these findings suggest a significant contribution of liquid-liquid phase separation to the processes involving TSPYL5, providing new insights into the role of this protein in the cell's molecular life.
Keywords: TSPYL5; fluorescence recovery after photobleaching (FRAP); intrinsically disordered proteins (IDPs); intrinsically disordered regions (IDRs); liquid–liquid phase separation (LLPS); protein–protein interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The stability of NPM1 oligomers regulated by acidic disordered regions controls the quality of liquid droplets.J Biochem. 2023 Oct 31;174(5):461-476. doi: 10.1093/jb/mvad061. J Biochem. 2023. PMID: 37540843
-
Liquid-Liquid Phase Separation by Intrinsically Disordered Protein Regions of Viruses: Roles in Viral Life Cycle and Control of Virus-Host Interactions.Int J Mol Sci. 2020 Nov 28;21(23):9045. doi: 10.3390/ijms21239045. Int J Mol Sci. 2020. PMID: 33260713 Free PMC article. Review.
-
In-Silico Analysis of pH-Dependent Liquid-Liquid Phase Separation in Intrinsically Disordered Proteins.Biomolecules. 2022 Jul 12;12(7):974. doi: 10.3390/biom12070974. Biomolecules. 2022. PMID: 35883530 Free PMC article.
-
Chaotic aging: intrinsically disordered proteins in aging-related processes.Cell Mol Life Sci. 2023 Aug 27;80(9):269. doi: 10.1007/s00018-023-04897-3. Cell Mol Life Sci. 2023. PMID: 37634152 Free PMC article.
-
The nucleolus from a liquid droplet perspective.J Biochem. 2021 Oct 11;170(2):153-162. doi: 10.1093/jb/mvab090. J Biochem. 2021. PMID: 34358306 Review.
References
-
- Qin S., Liu D., Kohli M., Wang L., Vedell P.T., Hillman D.W., Niu N., Yu J., Weinshilboum R.M., Wang L. TSPYL Family Regulates CYP17A1 and CYP3A4 Expression: Potential Mechanism Contributing to Abiraterone Response in Metastatic Castration-Resistant Prostate Cancer. Clin. Pharmacol. Ther. 2018;104:201–210. doi: 10.1002/cpt.907. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

