Precision Immunotherapy Utilizing Adapter CAR-T Cells (AdCAR-T) in Metastatic Breast Cancer Leads to Target Specific Lysis
- PMID: 38201595
- PMCID: PMC10778501
- DOI: 10.3390/cancers16010168
Precision Immunotherapy Utilizing Adapter CAR-T Cells (AdCAR-T) in Metastatic Breast Cancer Leads to Target Specific Lysis
Abstract
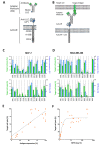
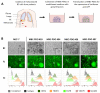
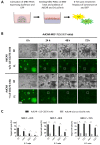
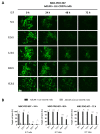
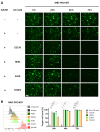
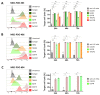
A frequent symptom of metastasized breast cancer (BC) includes the development of malignant pleural effusion (MPE), which contains malignant cells derived from the primary tumor site. The poor prognosis of MPE in metastasized BC indicates the necessity for dependable precision oncology and the importance of models representing the heterogenous nature of metastatic BC. In this study, we cultured MPE-derived metastatic tumor cells from four advanced BC patients using organoid technology. We assessed the expression of tumor-associated antigens on MPE-derived organoid lines by flow cytometry (FC). Based on an individual antigen expression pattern, patient-derived organoids were treated with adapter CAR-T cells (AdCAR-T) and biotinylated monoclonal antibodies targeting CD276, HER2, EGFR, TROP2, or EpCAM. Co-culture assays revealed specific organoid lysis by AdCAR-T depending on individual antigen expression patterns. Our results demonstrate that MPE-derived organoids can serve as a reliable tool for assessing the efficacy of AdCAR-T on metastatic BC in a patient-individualized manner. This approach could potentially be applied in a preclinical setting to instruct therapy decisions. Further, our study demonstrates the feasibility of precision immunotherapy utilizing AdCAR-T to target patient-individualized antigen patterns.
Keywords: adapter CAR-T cells; breast cancer; cancer biology; metastasis; organoid culture; patient-derived organoids; pleural effusion; precision immunotherapy.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Advancing Cancer Therapy Predictions with Patient-Derived Organoid Models of Metastatic Breast Cancer.Cancers (Basel). 2023 Jul 13;15(14):3602. doi: 10.3390/cancers15143602. Cancers (Basel). 2023. PMID: 37509265 Free PMC article.
-
Breast cancer organoids from malignant pleural effusion-derived tumor cells as an individualized medicine platform.In Vitro Cell Dev Biol Anim. 2021 May;57(5):510-518. doi: 10.1007/s11626-021-00563-9. Epub 2021 May 5. In Vitro Cell Dev Biol Anim. 2021. PMID: 33950403
-
Novel adapter CAR-T cell technology for precisely controllable multiplex cancer targeting.Oncoimmunology. 2021 Dec 2;10(1):2003532. doi: 10.1080/2162402X.2021.2003532. eCollection 2021. Oncoimmunology. 2021. PMID: 35686214 Free PMC article.
-
Breast cancer organoids and their applications for precision cancer immunotherapy.World J Surg Oncol. 2023 Oct 26;21(1):343. doi: 10.1186/s12957-023-03231-2. World J Surg Oncol. 2023. PMID: 37884976 Free PMC article. Review.
-
Updated Clinical Perspectives and Challenges of Chimeric Antigen Receptor-T Cell Therapy in Colorectal Cancer and Invasive Breast Cancer.Arch Immunol Ther Exp (Warsz). 2023 Aug 11;71(1):19. doi: 10.1007/s00005-023-00684-x. Arch Immunol Ther Exp (Warsz). 2023. PMID: 37566162 Review.
Cited by
-
UniCAR T-Cell Potency-A Matter of Affinity between Adaptor Molecules and Adaptor CAR T-Cells?Int J Mol Sci. 2024 Jun 30;25(13):7242. doi: 10.3390/ijms25137242. Int J Mol Sci. 2024. PMID: 39000348 Free PMC article.
-
The Transformative Role of 3D Culture Models in Triple-Negative Breast Cancer Research.Cancers (Basel). 2024 May 13;16(10):1859. doi: 10.3390/cancers16101859. Cancers (Basel). 2024. PMID: 38791938 Free PMC article. Review.
References
-
- Sorlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M.B., van de Rijn M., Jeffrey S.S., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. - DOI - PMC - PubMed
-
- Aurilio G., Disalvatore D., Pruneri G., Bagnardi V., Viale G., Curigliano G., Adamoli L., Munzone E., Sciandivasci A., De Vita F., et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur. J. Cancer. 2014;50:277–289. doi: 10.1016/j.ejca.2013.10.004. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

