Current Landscape of Immune Checkpoint Inhibitors for Metastatic Urothelial Carcinoma: Is There a Role for Additional T-Cell Blockade?
- PMID: 38201559
- PMCID: PMC10778285
- DOI: 10.3390/cancers16010131
Current Landscape of Immune Checkpoint Inhibitors for Metastatic Urothelial Carcinoma: Is There a Role for Additional T-Cell Blockade?
Abstract
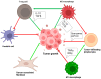
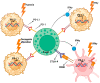
Urothelial carcinoma (UC) is the most common form of bladder cancer (BC) and is the variant with the most immunogenic response. This makes urothelial carcinoma an ideal candidate for immunotherapy with immune checkpoint inhibitors. Key immune checkpoint proteins PD-1 and CTLA-4 are frequently expressed on T-cells in urothelial carcinoma. The blockade of this immune checkpoint can lead to the reactivation of lymphocytes and augment the anti-tumor immune response. The only immune checkpoint inhibitors that are FDA-approved for metastatic urothelial carcinoma target the programmed death-1 receptor and its ligand (PD-1/PD-L1) axis. However, the overall response rate and progression-free survival rates of these agents are limited in this patient population. Therefore, there is a need to find further immune-bolstering treatment combinations that may positively impact survival for patients with advanced UC. In this review, the current immune checkpoint inhibition treatment landscape is explored with an emphasis on combination therapy in the form of PD-1/PD-L1 with CTLA-4 blockade. The investigation of the current literature on immune checkpoint inhibition found that preclinical data show a decrease in tumor volumes and size when PD-1/PD-L1 is blocked, and similar results were observed with CTLA-4 blockade. However, there are limited investigations evaluating the combination of CTLA-4 and PD-1/PD-L1 blockade. We anticipate this review to provide a foundation for a deeper experimental investigation into combination immune checkpoint inhibition therapy in metastatic urothelial carcinoma.
Keywords: CTLA-4; PD-1; bladder cancer; immune checkpoint inhibitor; urothelial carcinoma.
Conflict of interest statement
J.S.C. and S.T.N. are co-founders of Majestic Therapeutics, LLC. The other authors declare no conflicts of interest.
Figures

Similar articles
-
Phase 2 trial of tremelimumab in patients with metastatic urothelial cancer previously treated with programmed death 1/programmed death ligand 1 blockade.Cancer. 2024 May 1;130(9):1642-1649. doi: 10.1002/cncr.35179. Epub 2024 Jan 5. Cancer. 2024. PMID: 38180804 Clinical Trial.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
Role of Checkpoint Inhibition in Localized Bladder Cancer.Eur Urol Oncol. 2018 Aug;1(3):190-198. doi: 10.1016/j.euo.2018.05.002. Epub 2018 May 30. Eur Urol Oncol. 2018. PMID: 31102620
-
Combined Next-generation Sequencing and Flow Cytometry Analysis for an Anti-PD-L1 Partial Responder over Time: An Exploration of Mechanisms of PD-L1 Activity and Resistance in Bladder Cancer.Eur Urol Oncol. 2021 Feb;4(1):117-120. doi: 10.1016/j.euo.2019.01.017. Epub 2019 Feb 26. Eur Urol Oncol. 2021. PMID: 31411999
-
Novel immunotherapy approaches for metastatic urothelial and renal cell carcinoma.Asian J Urol. 2016 Oct;3(4):268-277. doi: 10.1016/j.ajur.2016.08.013. Epub 2016 Sep 6. Asian J Urol. 2016. PMID: 29264195 Free PMC article. Review.
Cited by
-
Protein-Based Predictive Biomarkers to Personalize Neoadjuvant Therapy for Bladder Cancer-A Systematic Review of the Current Status.Int J Mol Sci. 2024 Sep 13;25(18):9899. doi: 10.3390/ijms25189899. Int J Mol Sci. 2024. PMID: 39337385 Free PMC article.
-
Contemporary Molecular Markers for Predicting Systemic Treatment Response in Urothelial Bladder Cancer: A Narrative Review.Cancers (Basel). 2024 Sep 1;16(17):3056. doi: 10.3390/cancers16173056. Cancers (Basel). 2024. PMID: 39272913 Free PMC article. Review.
-
Identification of a circadian-based prognostic signature predicting cancer-associated fibroblasts infiltration and immunotherapy response in bladder cancer.Aging (Albany NY). 2024 Aug 30;16(17):12312-12334. doi: 10.18632/aging.206088. Epub 2024 Aug 30. Aging (Albany NY). 2024. PMID: 39216004 Free PMC article.
References
-
- National Cancer Institute . Bladder Cancer Stages—NCI. National Cancer Institute; Bethesda, MA, USA: 2023.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

