Super-Resolution Analysis of the Origins of the Elementary Events of ER Calcium Release in Dorsal Root Ganglion Neurons
- PMID: 38201242
- PMCID: PMC10778190
- DOI: 10.3390/cells13010038
Super-Resolution Analysis of the Origins of the Elementary Events of ER Calcium Release in Dorsal Root Ganglion Neurons
Abstract
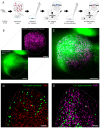
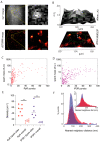
Coordinated events of calcium (Ca2+) released from the endoplasmic reticulum (ER) are key second messengers in excitable cells. In pain-sensing dorsal root ganglion (DRG) neurons, these events can be observed as Ca2+ sparks, produced by a combination of ryanodine receptors (RyR) and inositol 1,4,5-triphosphate receptors (IP3R1). These microscopic signals offer the neuronal cells with a possible means of modulating the subplasmalemmal Ca2+ handling, initiating vesicular exocytosis. With super-resolution dSTORM and expansion microscopies, we visualised the nanoscale distributions of both RyR and IP3R1 that featured loosely organised clusters in the subplasmalemmal regions of cultured rat DRG somata. We adapted a novel correlative microscopy protocol to examine the nanoscale patterns of RyR and IP3R1 in the locality of each Ca2+ spark. We found that most subplasmalemmal sparks correlated with relatively small groups of RyR whilst larger sparks were often associated with larger groups of IP3R1. These data also showed spontaneous Ca2+ sparks in <30% of the subplasmalemmal cell area but consisted of both these channel species at a 3.8-5 times higher density than in nonactive regions of the cell. Taken together, these observations reveal distinct patterns and length scales of RyR and IP3R1 co-clustering at contact sites between the ER and the surface plasmalemma that encode the positions and the quantity of Ca2+ released at each Ca2+ spark.
Keywords: calcium signalling; correlative microscopy; dSTORM; dorsal root ganglion neurons; expansion microscopy; inositol 1,4,5-trisphosphate receptor; ryanodine receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Ca2+ sparks and secretion in dorsal root ganglion neurons.Proc Natl Acad Sci U S A. 2005 Aug 23;102(34):12259-64. doi: 10.1073/pnas.0408494102. Epub 2005 Aug 15. Proc Natl Acad Sci U S A. 2005. PMID: 16103366 Free PMC article.
-
Correlative super-resolution analysis of cardiac calcium sparks and their molecular origins in health and disease.Open Biol. 2023 May;13(5):230045. doi: 10.1098/rsob.230045. Epub 2023 May 24. Open Biol. 2023. PMID: 37220792 Free PMC article.
-
3D dSTORM imaging reveals novel detail of ryanodine receptor localization in rat cardiac myocytes.J Physiol. 2019 Jan;597(2):399-418. doi: 10.1113/JP277360. Epub 2018 Nov 28. J Physiol. 2019. PMID: 30412283 Free PMC article.
-
Atrial local Ca2+ signaling and inositol 1,4,5-trisphosphate receptors.Prog Biophys Mol Biol. 2010 Sep;103(1):59-70. doi: 10.1016/j.pbiomolbio.2010.02.002. Epub 2010 Mar 1. Prog Biophys Mol Biol. 2010. PMID: 20193706 Review.
-
Structural insights into endoplasmic reticulum stored calcium regulation by inositol 1,4,5-trisphosphate and ryanodine receptors.Biochim Biophys Acta. 2015 Sep;1853(9):1980-91. doi: 10.1016/j.bbamcr.2014.11.023. Epub 2014 Nov 25. Biochim Biophys Acta. 2015. PMID: 25461839 Review.
References
-
- Miteva K.T., Pedicini L., Wilson L.A., Jayasinghe I., Slip R.G., Marszalek K., Gaunt H.J., Bartoli F., Deivasigamani S., Sobradillo D., et al. Rab46 integrates Ca2+ and histamine signaling to regulate selective cargo release from Weibel-Palade bodies. J. Cell Biol. 2019;218:2232–2246. doi: 10.1083/jcb.201810118. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

