Flammutoxin, a Degradation Product of Transepithelial Electrical Resistance-Decreasing Protein, Induces Reactive Oxygen Species and Apoptosis in HepG2 Cells
- PMID: 38201094
- PMCID: PMC10778570
- DOI: 10.3390/foods13010066
Flammutoxin, a Degradation Product of Transepithelial Electrical Resistance-Decreasing Protein, Induces Reactive Oxygen Species and Apoptosis in HepG2 Cells
Abstract
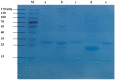
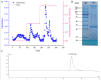
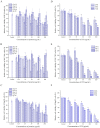
Proteins from Flammulina filiformis were prepared by sodium chloride extraction and fractionated by ammonium sulfate precipitation with increasing saturation degrees to obtain the protein fractions Ffsp-30, Ffsp-50, Ffsp-70, Ffsp-90, and Ffp-90. Among these protein fractions, Ffsp-50 possessed the most significant cytotoxic effect against three human gastrointestinal cancer cell lines, viz. HT-29, SGC-7901, and HepG2. SDS-PAGE and MALDI-TOF/TOF MS/MS analyses revealed that flammutoxin (FTX) was present as a dominating protein in Ffsp-50, which was further evidenced by HPLC-MS/MS determination. Furthermore, native FTX was purified from Ffsp-50 with a molecular weight of 26.78 kDa, exhibiting notable cytotoxicity against gastrointestinal cancer cell lines. Both Ffsp-50 and FTX exposure could enhance intercellular reactive oxygen species (ROS) generation and induce significant apoptosis in HepG2 cells. FTX was identified to be relatively conserved in basidiomycetes according to phylogenetic analysis, and its expression was highly upregulated in the primordium as well as the pileus of the fruiting body from the elongation and maturation stages, as compared with that in mycelium. Taken together, FTX could remarkably inhibit cell growth and induce ROS and apoptosis in HepG2 cells, potentially participating in the growth and development of the fruiting body. These findings from our investigation provided insight into the antigastrointestinal cancer activity of FTX, which could serve as a biological source of health-promoting and biomedical applications.
Keywords: Flammulina filiformis; apoptosis; flammutoxin; gastrointestinal cancer; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
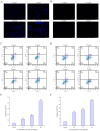
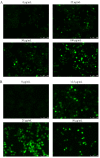

Similar articles
-
Combination of on-line desalting and HPLC-UV-ESI-MS for simultaneous detection and identification of FIP-fve and flammutoxin in Flammulina velutipes.J Food Drug Anal. 2018 Jul;26(3):1045-1053. doi: 10.1016/j.jfda.2017.12.004. Epub 2018 Jan 17. J Food Drug Anal. 2018. PMID: 29976397 Free PMC article.
-
Protein sequence analysis, cloning, and expression of flammutoxin, a pore-forming cytolysin from Flammulina velutipes. Maturation of dimeric precursor to monomeric active form by carboxyl-terminal truncation.J Biol Chem. 2004 Dec 24;279(52):54161-72. doi: 10.1074/jbc.M408783200. Epub 2004 Oct 15. J Biol Chem. 2004. PMID: 15489231
-
Application of thermal stability difference to remove flammutoxin in fungal immunomodulatory protein, FIP-fve, extract from Flammulina velutipes.J Food Drug Anal. 2018 Jul;26(3):1005-1014. doi: 10.1016/j.jfda.2017.12.010. Epub 2018 Jan 17. J Food Drug Anal. 2018. PMID: 29976393 Free PMC article.
-
Effect of a pore-forming protein derived from Flammulina velutipes on the Caco-2 intestinal epithelial cell monolayer.Biosci Biotechnol Biochem. 2004 Nov;68(11):2230-8. doi: 10.1271/bbb.68.2230. Biosci Biotechnol Biochem. 2004. PMID: 15564659
-
Reactive Oxygen Species Distribution Involved in Stipe Gradient Elongation in the Mushroom Flammulina filiformis.Cells. 2022 Jun 11;11(12):1896. doi: 10.3390/cells11121896. Cells. 2022. PMID: 35741023 Free PMC article.
References
-
- Chen J., Li J.M., Tang Y.J., Ma K., Li B., Zeng X., Liu X.B., Li Y., Yang Z.L., Xu W.N., et al. Genome-wide analysis and prediction of genes involved in the biosynthesis of polysaccharides and bioactive secondary metabolites in high-temperature-tolerant wild Flammulina filiformis. BMC Genom. 2020;21:719. doi: 10.1186/s12864-020-07108-6. - DOI - PMC - PubMed
-
- Ding Y., Chen S.T., Wang H.L., Li S.L., Ma C.Y., Wang J.M., Cui L.L. Identification of secondary metabolites in Flammulina velutipes by UPLC-Q-Exactive-Orbitrap MS. J. Food Qual. 2021;2021:4103952. doi: 10.1155/2021/4103952. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

