Clinical human metapneumovirus isolates show distinct pathogenesis and inflammatory profiles but similar CD8+ T cell impairment
- PMID: 38197640
- PMCID: PMC10826344
- DOI: 10.1128/msphere.00570-23
Clinical human metapneumovirus isolates show distinct pathogenesis and inflammatory profiles but similar CD8+ T cell impairment
Abstract
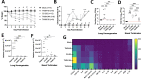
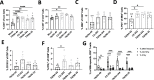
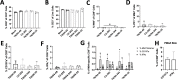
Human metapneumovirus (HMPV) is a negative-sense single-stranded RNA virus in the Pneumoviridae family and a leading cause of acute upper and lower respiratory infections, particularly in children, immunocompromised patients, and the elderly. Although nearly every person is infected with HMPV during early childhood, re-infections occur often, highlighting difficulty in building long-term immunity. Inflammatory responses, including PD-1-mediated impairment of virus-specific CD8+ T cells (TCD8), contribute to HMPV disease severity. HMPV strains are divided into four lineages: A1, A2, B1, and B2. However, little is known about immune responses to different viral subtypes. Here, we characterize responses to four HMPV clinical isolates-TN/94-344 (A1), TN/94-49 (A2), C2-202 (B1), and TN/96-35 (B2)-in vivo in C57BL/6 (B6) mice. TN/94-49 was avirulent, while TN/94-344, C2-202, and TN/96-35 showed varying degrees of weight loss and clinical disease. Differences in disease did not correlate to virus burden in upper or lower tracts. TN/94-49 HMPV exhibited highest nose titers and delayed lung clearance. Cytokine profiles differed between HMPV isolates, with TN/96-35 inducing the broadest lung inflammatory cytokines. TN/96-35 also showed lower HMPV burden and less weight loss than other virulent isolates, suggesting a more efficient antiviral response. Interestingly, disease correlated with higher expression of T-cell chemoattractant CXCL9. All isolates elicited PD-1 upregulation and decreased IFNγ and CD107a expression in virus-specific TCD8, with little difference between HMPV subtypes. This work uncovers previously uncharacterized variations in immune responses to clinical HMPV isolates of different lineages.IMPORTANCEThis study extensively explored differences in T-cell-mediated immunity between human metapneumovirus (HMPV) clinical isolates. Much existing HMPV research has been done with strains passaged extensively in cell lines, likely acquiring mutations advantageous to in vitro replication. Clinical isolates are collected directly from human patients and have undergone <10 passages, serving as more physiologically relevant models of HMPV infection. Additionally, existing animal studies of HMPV disease mainly focus on lung pathogenesis, while HMPV infects both upper and lower airways of humans. This work highlights distinct differences in HMPV burden in upper and lower tracts between clinical isolates. Lastly, this study uniquely explores differences in host immunity between all four HMPV genetic lineages. The predominant HMPV subtype in circulation varies seasonally; thus, understanding host responses to all subgroups is critical for developing effective HMPV vaccines.
Keywords: airway immunity; human metapneumovirus; respiratory infection.
Conflict of interest statement
J.V.W. serves on the Scientific Advisory Board of Quidel and an Independent Data Monitoring Committee for GlaxoSmithKline, neither activity involved with the work under consideration. All other authors declare no conflicts of interest.
Figures

Similar articles
-
IFN-λ drives distinct lung immune landscape changes and antiviral responses in human metapneumovirus infection.mBio. 2024 May 8;15(5):e0055024. doi: 10.1128/mbio.00550-24. Epub 2024 Mar 26. mBio. 2024. PMID: 38530032 Free PMC article.
-
Lung CD8+ T Cell Impairment Occurs during Human Metapneumovirus Infection despite Virus-Like Particle Induction of Functional CD8+ T Cells.J Virol. 2015 Sep;89(17):8713-26. doi: 10.1128/JVI.00670-15. Epub 2015 Jun 10. J Virol. 2015. PMID: 26063431 Free PMC article.
-
Role of type I interferon signaling in human metapneumovirus pathogenesis and control of viral replication.J Virol. 2015 Apr;89(8):4405-20. doi: 10.1128/JVI.03275-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653440 Free PMC article.
-
Human Metapneumovirus: Mechanisms and Molecular Targets Used by the Virus to Avoid the Immune System.Front Immunol. 2018 Oct 24;9:2466. doi: 10.3389/fimmu.2018.02466. eCollection 2018. Front Immunol. 2018. PMID: 30405642 Free PMC article. Review.
-
Aberrant T cell immunity triggered by human Respiratory Syncytial Virus and human Metapneumovirus infection.Virulence. 2017 Aug 18;8(6):685-704. doi: 10.1080/21505594.2016.1265725. Epub 2016 Dec 2. Virulence. 2017. PMID: 27911218 Free PMC article. Review.
Cited by
-
IFN-λ drives distinct lung immune landscape changes and antiviral responses in human metapneumovirus infection.mBio. 2024 May 8;15(5):e0055024. doi: 10.1128/mbio.00550-24. Epub 2024 Mar 26. mBio. 2024. PMID: 38530032 Free PMC article.
References
-
- Williams JV, Wang CK, Yang C-F, Tollefson SJ, House FS, Heck JM, Chu M, Brown JB, Lintao LD, Quinto JD, Chu D, Spaete RR, Edwards KM, Wright PF, Crowe JE. 2006. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis 193:387–395. doi:10.1086/499274 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

