Effect of injectable calcium alginate-amelogenin hydrogel on macrophage polarization and promotion of jawbone osteogenesis
- PMID: 38196914
- PMCID: PMC10774865
- DOI: 10.1039/d3ra05046g
Effect of injectable calcium alginate-amelogenin hydrogel on macrophage polarization and promotion of jawbone osteogenesis
Abstract
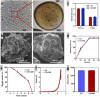
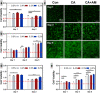
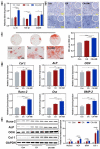
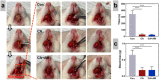
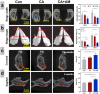
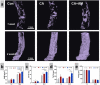
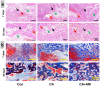
Due to persistent inflammation and limited osteogenesis, jawbone defects present a considerable challenge in regenerative medicine. Amelogenin, a major protein constituent of the developing enamel matrix, demonstrates promising capabilities in inducing regeneration of periodontal supporting tissues and exerting immunomodulatory effects. These properties render it a potential therapeutic agent for enhancing jawbone osteogenesis. Nevertheless, its clinical application is hindered by the limitations of monotherapy and its rapid release characteristics, which compromise its efficacy and delivery efficiency. In this context, calcium alginate hydrogel, recognized for its superior physicochemical properties and biocompatibility, emerges as a candidate for developing a synergistic bioengineered drug delivery system. This study describes the synthesis of an injectable calcium amelogenin/calcium alginate hydrogel using calcium alginate loaded with amelogenin. We comprehensively investigated its physical properties, its role in modulating the immunological environment conducive to bone healing, and its osteogenic efficacy in areas of jawbone defects. Our experimental findings indicate that this synthesized composite hydrogel possesses desirable mechanical properties such as injectability, biocompatibility, and biodegradability. Furthermore, it facilitates jawbone formation by regulating the bone-healing microenvironment and directly inducing osteogenesis. This research provides novel insights into the development of bone-tissue regeneration materials, potentially advancing their clinical application.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Wang X. Li C. He T. Q. Zheng W. H. Liu W. Zhang Y. Y. Chen X. L. Zhou Y. Q. Shui C. Y. Ning Y. D. Cai Y. C. Jiang J. Sun R. H. Wang W. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2021;56:89–92. - PubMed
LinkOut - more resources
Full Text Sources

