Neurons cytoskeletal architecture remodeling during the replication cycle of mouse coronavirus MHV-JHM: a morphological in vitro study
- PMID: 38195523
- PMCID: PMC10775625
- DOI: 10.1186/s12917-023-03813-y
Neurons cytoskeletal architecture remodeling during the replication cycle of mouse coronavirus MHV-JHM: a morphological in vitro study
Abstract
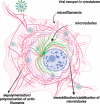
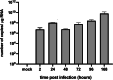
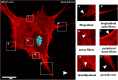
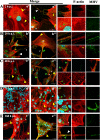
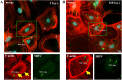
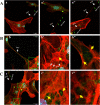
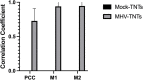
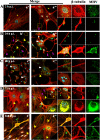
Nowadays, the population is still struggling with a post-COVID19 syndrome known as long COVID, including a broad spectrum of neurological problems. There is an urgent need for a better understanding and exploration of the mechanisms of coronavirus neurotropism. For this purpose, the neurotropic strain of mouse hepatitis virus (MHV-JHM) originating from the beta-coronavirus genus, the same as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been used. The role of the cytoskeleton during virus replication in neurons in vitro was determined to understand the mechanisms of MHV-JHM neuroinfection. We have described for the first time the changes of actin filaments during MHV-JHM infection. We also observed productive replication of MHV-JHM in neurons during 168 h p.i. and syncytial cytopathic effect. We discovered that the MHV-JHM strain modulated neuronal cytoskeleton during infection, which were manifested by: (i) condensation of actin filaments in the cortical layer of the cytoplasm, (ii) formation of microtubule cisternae structures containing viral antigen targeting viral replication site (iii) formation of tunneling nanotubes used by MHV-JHM for intercellular transport. Additionally, we demonstrated that the use of cytoskeletal inhibitors have reduced virus replication in neurons, especially noscapine and nocodazole, the microtubule shortening factors.
Keywords: Actin filaments; MHV-JHM; Microtubules; Neurons; Neurotropism.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
The nsp1, nsp13, and M proteins contribute to the hepatotropism of murine coronavirus JHM.WU.J Virol. 2015 Apr;89(7):3598-609. doi: 10.1128/JVI.03535-14. Epub 2015 Jan 14. J Virol. 2015. PMID: 25589656 Free PMC article.
-
Responses of mice to murine coronavirus immunization.Arch Virol. 1992;125(1-4):39-52. doi: 10.1007/BF01309627. Arch Virol. 1992. PMID: 1322658 Free PMC article.
-
Mouse hepatitis virus strain JHM infects a human hepatocellular carcinoma cell line.Virology. 1999 Nov 25;264(2):398-409. doi: 10.1006/viro.1999.9984. Virology. 1999. PMID: 10562501 Free PMC article.
-
Murine coronavirus neuropathogenesis: determinants of virulence.J Neurovirol. 2010 Nov;16(6):427-34. doi: 10.3109/13550284.2010.529238. Epub 2010 Nov 12. J Neurovirol. 2010. PMID: 21073281 Free PMC article. Review.
-
Of Mice and Men: The Coronavirus MHV and Mouse Models as a Translational Approach to Understand SARS-CoV-2.Viruses. 2020 Aug 12;12(8):880. doi: 10.3390/v12080880. Viruses. 2020. PMID: 32806708 Free PMC article. Review.
Cited by
-
Antiviral Activity of Graphene Oxide-Silver Nanocomposites Against Murine Betacoronavirus.Int J Nanomedicine. 2024 Sep 4;19:9009-9033. doi: 10.2147/IJN.S473448. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39246425 Free PMC article.
-
Neurological, psychological, psychosocial complications of long-COVID and their management.Neurol Sci. 2025 Jan;46(1):1-23. doi: 10.1007/s10072-024-07854-5. Epub 2024 Nov 9. Neurol Sci. 2025. PMID: 39516425 Free PMC article. Review.
References
-
- Kubitschke H, Schnauss J, Nnetu KD, Warmt E, Stange R, Kaes J. Actin and microtubule networks contribute differently to cell response for small and large strains. New J Phys. 2017;19(9):093003. doi: 10.1088/1367-2630/aa7658. - DOI
-
- Kłyszejko-Stefanowicz L. Cytobiochemia – biochemia niektórych struktur komórkowych. 3. Warsaw: PWN SA; 2015. pp. 58–62.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

