Sex differences of NF-κB-targeted therapy for mitigating osteoporosis associated with chronic inflammation of bone
- PMID: 38194999
- PMCID: PMC10776185
- DOI: 10.1302/2046-3758.131.BJR-2023-0040.R3
Sex differences of NF-κB-targeted therapy for mitigating osteoporosis associated with chronic inflammation of bone
Abstract
Aims: Transcription factor nuclear factor kappa B (NF-κB) plays a major role in the pathogenesis of chronic inflammatory diseases in all organ systems. Despite its importance, NF-κB targeted drug therapy to mitigate chronic inflammation has had limited success in preclinical studies. We hypothesized that sex differences affect the response to NF-κB treatment during chronic inflammation in bone. This study investigated the therapeutic effects of NF-κB decoy oligodeoxynucleotides (ODN) during chronic inflammation in male and female mice.
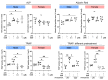
Methods: We used a murine model of chronic inflammation induced by continuous intramedullary delivery of lipopolysaccharide-contaminated polyethylene particles (cPE) using an osmotic pump. Specimens were evaluated using micro-CT and histomorphometric analyses. Sex-specific osteogenic and osteoclastic differentiation potentials were also investigated in vitro, including alkaline phosphatase, Alizarin Red, tartrate-resistant acid phosphatase staining, and gene expression using reverse transcription polymerase chain reaction (RT-PCR).
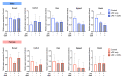
Results: Local delivery of NF-κB decoy ODN in vivo increased osteogenesis in males, but not females, in the presence of chronic inflammation induced by cPE. Bone resorption activity was decreased in both sexes. In vitro osteogenic and osteoclastic differentiation assays during inflammatory conditions did not reveal differences among the groups. Receptor activator of nuclear factor kappa Β ligand (Rankl) gene expression by osteoblasts was significantly decreased only in males when treated with ODN.
Conclusion: We demonstrated that NF-κB decoy ODN increased osteogenesis in male mice and decreased bone resorption activity in both sexes in preclinical models of chronic inflammation. NF-κB signalling could be a therapeutic target for chronic inflammatory diseases involving bone, especially in males.
© 2024 Goodman et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
NF-κB decoy oligodeoxynucleotide mitigates wear particle-associated bone loss in the murine continuous infusion model.Acta Biomater. 2016 Sep 1;41:273-81. doi: 10.1016/j.actbio.2016.05.038. Epub 2016 May 31. Acta Biomater. 2016. PMID: 27260104 Free PMC article.
-
NF-κB Decoy ODN-Loaded Poly(Lactic-co-glycolic Acid) Nanospheres Inhibit Alveolar Ridge Resorption.Int J Mol Sci. 2023 Feb 12;24(4):3699. doi: 10.3390/ijms24043699. Int J Mol Sci. 2023. PMID: 36835111 Free PMC article.
-
NF-κB decoy oligodeoxynucleotide inhibits wear particle-induced inflammation in a murine calvarial model.J Biomed Mater Res A. 2015 Dec;103(12):3872-8. doi: 10.1002/jbm.a.35532. Epub 2015 Jul 14. J Biomed Mater Res A. 2015. PMID: 26123702 Free PMC article.
-
Suppression of NF-κB-induced chronic inflammation mitigates inflammatory osteolysis in the murine continuous polyethylene particle infusion model.J Biomed Mater Res A. 2021 Oct;109(10):1828-1839. doi: 10.1002/jbm.a.37175. Epub 2021 Mar 28. J Biomed Mater Res A. 2021. PMID: 33779115 Free PMC article.
-
Sex differences in the therapeutic effect of unaltered versus NFκB sensing IL-4 over-expressing mesenchymal stromal cells in a murine model of chronic inflammatory bone loss.Front Bioeng Biotechnol. 2022 Aug 15;10:962114. doi: 10.3389/fbioe.2022.962114. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36046680 Free PMC article.
References
-
- Kim MH, Choi LY, Chung JY, Kim E-J, Yang WM. Auraptene ameliorates osteoporosis by inhibiting RANKL/NFATc1 pathway-mediated bone resorption based on network pharmacology and experimental evaluation. Bone Joint Res. 2022;11(5):304–316. doi: 10.1302/2046-3758.115.BJR-2021-0380.R1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

