Achaete-scute family bHLH transcription factor 2 activation promotes hepatoblastoma progression
- PMID: 38183173
- PMCID: PMC10921009
- DOI: 10.1111/cas.16051
Achaete-scute family bHLH transcription factor 2 activation promotes hepatoblastoma progression
Abstract
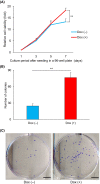
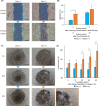
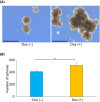
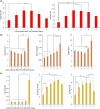
Achaete-scute family bHLH transcription factor 2 (ASCL2) is highly expressed in hepatoblastoma (HB) tissues, but its role remains unclear. Thus, biological changes in the HB cell line HepG2 in response to induced ASCL2 expression were assessed. ASCL2 expression was induced in HepG2 cells using the Tet-On 3G system, which includes doxycycline. Cell viability, proliferation activity, mobility, and stemness were evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, colony-formation, migration, invasion, and sphere-formation assays. Quantitative reverse-transcription polymerase chain reaction was used to assess the expression of markers for proliferation (CCND1 and MYC), epithelial-mesenchymal transition (EMT; SNAI1, TWIST1, and ZEB1), mesenchymal-epithelial transition (CDH1), and stemness (KLF4, POU5F1, and SOX9). Compared with the non-induced HepG2 cells, cells with induced ASCL2 expression showed significant increases in viability, colony number, migration area (%), and sphere number on days 7, 14, 8, and 7, respectively, and invasion area (%) after 90 h. Furthermore, induction of ASCL2 expression significantly upregulated CCND1, MYC, POU5F1, SOX9, and KLF4 expression on days 2, 2, 3, 3, and 5, respectively, and increased the ratios of SNAI1, TWIST1, and ZEB1 to CDH1 on day 5. ASCL2 promoted the formation of malignant phenotypes in HepG2 cells, which may be correlated with the upregulation of the Wnt signaling pathway-, EMT-, and stemness-related genes. ASCL2 activation may therefore be involved in the progression of HB.
Keywords: Wnt signaling pathway; basic helix-loop-helix transcription factor; growth; hepatoblastoma; phenotype.
© 2024 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
MicroRNA-200 (miR-200) cluster regulation by achaete scute-like 2 (Ascl2): impact on the epithelial-mesenchymal transition in colon cancer cells.J Biol Chem. 2014 Dec 26;289(52):36101-15. doi: 10.1074/jbc.M114.598383. Epub 2014 Nov 4. J Biol Chem. 2014. PMID: 25371200 Free PMC article.
-
HIF-1α/Ascl2/miR-200b regulatory feedback circuit modulated the epithelial-mesenchymal transition (EMT) in colorectal cancer cells.Exp Cell Res. 2017 Nov 15;360(2):243-256. doi: 10.1016/j.yexcr.2017.09.014. Epub 2017 Sep 9. Exp Cell Res. 2017. PMID: 28899657
-
WiNTRLINC1/ASCL2/c-Myc Axis Characteristics of Colon Cancer with Differentiated Histology at Young Onset and Essential for Cell Viability.Ann Surg Oncol. 2019 Dec;26(13):4826-4834. doi: 10.1245/s10434-019-07780-3. Epub 2019 Sep 23. Ann Surg Oncol. 2019. PMID: 31549316
-
Small cell lung cancer, an epithelial to mesenchymal transition (EMT)-like cancer: significance of inactive Notch signaling and expression of achaete-scute complex homologue 1.Hum Cell. 2017 Jan;30(1):1-10. doi: 10.1007/s13577-016-0149-3. Epub 2016 Oct 26. Hum Cell. 2017. PMID: 27785690 Review.
-
Hes1: a key role in stemness, metastasis and multidrug resistance.Cancer Biol Ther. 2015;16(3):353-9. doi: 10.1080/15384047.2015.1016662. Cancer Biol Ther. 2015. PMID: 25781910 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

