Development of an integrated sample amplification control for salivary point-of-care pathogen testing
- PMID: 38182338
- PMCID: PMC10860388
- DOI: 10.1016/j.aca.2023.342072
Development of an integrated sample amplification control for salivary point-of-care pathogen testing
Abstract
Background: The COVID-19 pandemic has led to a rise in point-of-care (POC) and home-based tests, but concerns over usability, accuracy, and effectiveness have arisen. The incorporation of internal amplification controls (IACs), essential control for translational POC diagnostics, could mitigate false-negative and false-positive results due to sample matrix interference or inhibition. Although emerging POC nucleic acid amplification tests (NAATs) for detecting SARS-CoV-2 show impressive analytical sensitivity in the lab, the assessment of clinical accuracy with IACs is often overlooked. In some cases, the IACs were run spatially, complicating assay workflow. Therefore, the multiplex assay for pathogen and IAC is needed.
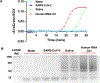
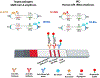
Results: We developed a one-pot duplex reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) assay for saliva samples, a non-invasive and simple collected specimen for POC NAATs. The ORF1ab gene of SARS-CoV-2 was used as a target and a human 18S ribosomal RNA in human saliva was employed as an IAC to ensure clinical reliability of the RT-LAMP assay. The optimized assay could detect SARS-CoV-2 viral particles down to 100 copies/μL of saliva within 30 min without RNA extraction. The duplex RT-LAMP for SARS-CoV-2 and IAC is successfully amplified in the same reaction without cross-reactivity. The valid results were easily visualized in triple-line lateral flow immunoassay, in which two lines (flow control and IAC lines) represent valid negative results and three lines (flow control, IAC, and test line) represent valid positive results. This duplex assay demonstrated a clinical sensitivity of 95%, specificity of 100%, and accuracy of 96% in 30 clinical saliva samples.
Significance: IACs play a crucial role in ensuring user confidence with respect to the accuracy and reliability of at-home and POC molecular diagnostics. We demonstrated the multiplex capability of SARS-COV-2 and human18S ribosomal RNA RT-LAMP without complicating assay design. This generic platform can be extended in a similar manner to include human18S ribosomal RNA IACs into different clinical sample matrices.
Keywords: Diagnostic; Internal amplification control; Lateral flow immunoassay; Reverse transcription loop-mediated isothermal amplification; SARS-CoV-2; Saliva.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest Jacqueline C. Linnes is co-founder of EverTrue LLC, a diagnostics company developing paper-based POC NAATs, and co-founder of OmniVis Inc. NAAT company developing POC diagnostics. Navaporn Sritong, Winston Wei Ngo, and Karin F. K. Ejendal have declared that they have no competing interests.
Figures


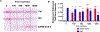
Update of
-
Development of an Integrated Sample Amplification Control for Salivary Point-of-Care Pathogen Testing.medRxiv [Preprint]. 2023 Oct 3:2023.10.03.23296477. doi: 10.1101/2023.10.03.23296477. medRxiv. 2023. Update in: Anal Chim Acta. 2024 Jan 25;1287:342072. doi: 10.1016/j.aca.2023.342072. PMID: 37873363 Free PMC article. Updated. Preprint.
Similar articles
-
Development of an Integrated Sample Amplification Control for Salivary Point-of-Care Pathogen Testing.medRxiv [Preprint]. 2023 Oct 3:2023.10.03.23296477. doi: 10.1101/2023.10.03.23296477. medRxiv. 2023. Update in: Anal Chim Acta. 2024 Jan 25;1287:342072. doi: 10.1016/j.aca.2023.342072. PMID: 37873363 Free PMC article. Updated. Preprint.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article. Review.
-
Highly sensitive extraction-free saliva-based molecular assay for rapid diagnosis of SARS-CoV-2.J Clin Microbiol. 2024 Jun 12;62(6):e0060024. doi: 10.1128/jcm.00600-24. Epub 2024 May 24. J Clin Microbiol. 2024. PMID: 38785448 Free PMC article.
-
RETRACTED: Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial.Int J Antimicrob Agents. 2020 Jul;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. Epub 2020 Mar 20. Int J Antimicrob Agents. 2020. Retraction in: Int J Antimicrob Agents. 2025 Jan;65(1):107416. doi: 10.1016/j.ijantimicag.2024.107416. PMID: 32205204 Free PMC article. Retracted. Clinical Trial.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
References
-
- Figueroa C, Johnson C, Ford N, Sands A, Dalal S, Meurant R, Prat I, Hatzold K, Urassa W, Baggaley R, Reliability of HIV rapid diagnostic tests for self-testing compared with testing by health-care workers: a systematic review and meta-analysis, Lancet HIV. 5 (2018) e277–e290. 10.1016/S2352-3018(18)30044-4. - DOI - PMC - PubMed
-
- Class II Special Controls Guideline: In Vitro Diagnostic Devices for Bacillus spp. Detection, (n.d.).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

