The impact of prior SARS-CoV-2 infection on host inflammatory cytokine profiles in patients with TB or other respiratory diseases
- PMID: 38179046
- PMCID: PMC10764540
- DOI: 10.3389/fimmu.2023.1292486
The impact of prior SARS-CoV-2 infection on host inflammatory cytokine profiles in patients with TB or other respiratory diseases
Abstract
Background: Tuberculosis (TB) and COVID-19 are the two leading causes of infectious disease mortality worldwide, and their overlap is likely frequent and inevitable. Previous research has shown increased mortality in TB/COVID-coinfected individuals, and emerging evidence suggests that COVID-19 may increase susceptibility to TB. However, the immunological mechanisms underlying these interactions remain unclear. In this study, we aimed to elucidate the impact of prior or concurrent COVID-19 infection on immune profiles of TB patients and those with other respiratory diseases (ORD).
Methods: Serum and nasopharyngeal samples were collected from 161 Gambian adolescents and adults with either TB or an ORD. Concurrent COVID-19 infection was determined by PCR, while prior COVID-19 was defined by antibody seropositivity. Multiplex cytokine immunoassays were used to quantify 27 cytokines and chemokines in patient serum samples at baseline, and throughout treatment in TB patients.
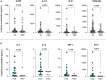
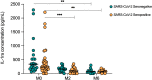
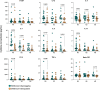
Results: Strikingly, TB and ORD patients with prior COVID-19 infection were found to have significantly reduced expression of several cytokines, including IL-1β, TNF-α and IL-7, compared to those without (p<0.035). Moreover, at month-six of anti-TB treatment, seropositive patients had lower serum Basic FGF (p=0.0115), IL-1β (p=0.0326) and IL-8 (p=0.0021) than seronegative. TB patients with acute COVID-19 coinfection had lower levels of IL-8, IL-13, TNF-α and IP-10 than TB-only patients, though these trends did not reach significance (p>0.035).
Conclusions: Our findings demonstrate that COVID-19 infection alters the subsequent response to TB and ORDs, potentially contributing to pathogenesis. Further work is necessary to determine whether COVID-19 infection accelerates TB disease progression, though our results experimentally support this hypothesis.
Keywords: COVID-19; cytokines; inflammatory profiles; respiratory disease; tuberculosis.
Copyright © 2023 Cottam, Manneh, Gindeh, Sillah, Cham, Mendy, Barry, Coker, Daffeh, Badjie, Barry, Owolabi, Winter, Walzl and Sutherland.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Characterization of the immune impairment of patients with tuberculosis and COVID-19 coinfection.Int J Infect Dis. 2023 May;130 Suppl 1:S34-S42. doi: 10.1016/j.ijid.2023.03.021. Epub 2023 Mar 21. Int J Infect Dis. 2023. PMID: 36944383 Free PMC article.
-
TB and COVID-19: An Exploration of the Characteristics and Resulting Complications of Co-infection.Front Biosci (Schol Ed). 2022 Mar 1;14(1):6. doi: 10.31083/j.fbs1401006. Front Biosci (Schol Ed). 2022. PMID: 35320917 Free PMC article. Review.
-
High levels of pro-inflammatory SARS-CoV-2-specific biomarkers revealed by in vitro whole blood cytokine release assay (CRA) in recovered and long-COVID-19 patients.PLoS One. 2023 Apr 5;18(4):e0283983. doi: 10.1371/journal.pone.0283983. eCollection 2023. PLoS One. 2023. PMID: 37018291 Free PMC article.
-
Characterization of the inflammatory response to COVID-19 illness in pregnancy.Cytokine. 2023 Oct;170:156319. doi: 10.1016/j.cyto.2023.156319. Epub 2023 Aug 4. Cytokine. 2023. PMID: 37544133
-
Pathogenesis of SARS-CoV-2 and Mycobacterium tuberculosis Coinfection.Front Immunol. 2022 Jun 16;13:909011. doi: 10.3389/fimmu.2022.909011. eCollection 2022. Front Immunol. 2022. PMID: 35784278 Free PMC article. Review.
Cited by
-
Immunoprofiles of COVID-19 uniquely differentiated from other viruses: A machine learning investigation of multiplex immunoassay data.PNAS Nexus. 2024 Aug 7;3(8):pgae327. doi: 10.1093/pnasnexus/pgae327. eCollection 2024 Aug. PNAS Nexus. 2024. PMID: 39157461 Free PMC article.
References
-
- World Health Organization . Global Tuberculosis Report 2022 (2022). Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa... (Accessed May 15 2023).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

