Cholinergic signaling via the α7 nicotinic acetylcholine receptor regulates the migration of monocyte-derived macrophages during acute inflammation
- PMID: 38178134
- PMCID: PMC10765732
- DOI: 10.1186/s12974-023-03001-7
Cholinergic signaling via the α7 nicotinic acetylcholine receptor regulates the migration of monocyte-derived macrophages during acute inflammation
Abstract
Background: The involvement of the autonomic nervous system in the regulation of inflammation is an emerging concept with significant potential for clinical applications. Recent studies demonstrate that stimulating the vagus nerve activates the cholinergic anti-inflammatory pathway that inhibits pro-inflammatory cytokines and controls inflammation. The α7 nicotinic acetylcholine receptor (α7nAChR) on macrophages plays a key role in mediating cholinergic anti-inflammatory effects through a downstream intracellular mechanism involving inhibition of NF-κB signaling, which results in suppression of pro-inflammatory cytokine production. However, the role of the α7nAChR in the regulation of other aspects of the immune response, including the recruitment of monocytes/macrophages to the site of inflammation remained poorly understood.
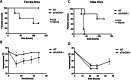
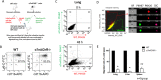
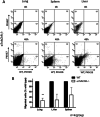
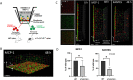
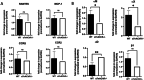
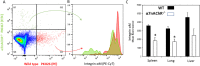
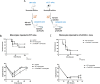
Results: We observed an increased mortality in α7nAChR-deficient mice (compared with wild-type controls) in mice with endotoxemia, which was paralleled with a significant reduction in the number of monocyte-derived macrophages in the lungs. Corroborating these results, fluorescently labeled α7nAChR-deficient monocytes adoptively transferred to WT mice showed significantly diminished recruitment to the inflamed tissue. α7nAChR deficiency did not affect monocyte 2D transmigration across an endothelial monolayer, but it significantly decreased the migration of macrophages in a 3D fibrin matrix. In vitro analysis of major adhesive receptors (L-selectin, β1 and β2 integrins) and chemokine receptors (CCR2 and CCR5) revealed reduced expression of integrin αM and αX on α7nAChR-deficient macrophages. Decreased expression of αMβ2 was confirmed on fluorescently labeled, adoptively transferred α7nAChR-deficient macrophages in the lungs of endotoxemic mice, indicating a potential mechanism for α7nAChR-mediated migration.
Conclusions: We demonstrate a novel role for the α7nAChR in mediating macrophage recruitment to inflamed tissue, which indicates an important new aspect of the cholinergic regulation of immune responses and inflammation.
Keywords: Cholinergic anti-inflammatory pathway; Endotoxemia; Macrophage; Migration; Sepsis; α7nAChR.
© 2023. The Author(s).
Conflict of interest statement
The authors declare they have no competing interests.
Figures

Similar articles
-
Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling.Mol Med. 2008 Sep-Oct;14(9-10):567-74. doi: 10.2119/2008-00079.Parrish. Mol Med. 2008. PMID: 18584048 Free PMC article.
-
Nicotinic acetylcholine receptor α7 subunit improves energy homeostasis and inhibits inflammation in nonalcoholic fatty liver disease.Metabolism. 2018 Feb;79:52-63. doi: 10.1016/j.metabol.2017.11.002. Epub 2017 Nov 10. Metabolism. 2018. PMID: 29129819
-
A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen.Gut. 2014 Jun;63(6):938-48. doi: 10.1136/gutjnl-2013-304676. Epub 2013 Aug 8. Gut. 2014. PMID: 23929694
-
The Role of α7nAChR-Mediated Cholinergic Anti-inflammatory Pathway in Immune Cells.Inflammation. 2021 Jun;44(3):821-834. doi: 10.1007/s10753-020-01396-6. Epub 2021 Jan 6. Inflammation. 2021. PMID: 33405021 Review.
-
Neuroimmune nexus in the pathophysiology and therapy of inflammatory disorders: Role of α7 nicotinic acetylcholine receptors.Pharmacol Res. 2023 May;191:106758. doi: 10.1016/j.phrs.2023.106758. Epub 2023 Apr 5. Pharmacol Res. 2023. PMID: 37028776 Review.
Cited by
-
Dysregulated brain-gut axis in the setting of traumatic brain injury: review of mechanisms and anti-inflammatory pharmacotherapies.J Neuroinflammation. 2024 May 10;21(1):124. doi: 10.1186/s12974-024-03118-3. J Neuroinflammation. 2024. PMID: 38730498 Free PMC article. Review.
-
Mechanisms underlying delirium in patients with critical illness.Front Aging Neurosci. 2024 Sep 26;16:1446523. doi: 10.3389/fnagi.2024.1446523. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39391586 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

