The blockade of the TGF-β pathway alleviates abnormal glucose and lipid metabolism of lipodystrophy not obesity
- PMID: 38174807
- PMCID: PMC10765454
- DOI: 10.1002/prp2.1160
The blockade of the TGF-β pathway alleviates abnormal glucose and lipid metabolism of lipodystrophy not obesity
Abstract
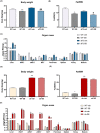
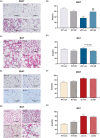
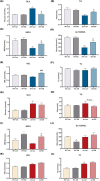
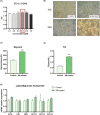
TGF-β is thought to be involved in the physiological functions of early organ development and pathological changes in substantial organ fibrosis, while studies around adipose tissue function and systemic disorders of glucolipid metabolism are still scarce. In this investigation, two animal models, aP2-SREBP-1c mice and ob/ob mice, were used. TGF-β pathway showed up-regulated in the inguinal white adipose tissue (iWAT) of the two models. SB431542, a TGF-β inhibitor, successfully increased inguinal white adipocyte size by more than 1.5 times and decreased the weight of Peripheral organs including liver, Spleen and Kidney to 73.05%/62.18%/73.23% of pre-administration weights. The iWAT showed elevated expression of GLUTs and lipases, followed by a recovery of circulation GLU, TG, NEFA, and GLYCEROL to the wild-type levels in aP2-SREBP-1c mice. In contrast, TGF-β inhibition did not have similar effects on that of ob/ob mice. In vitro, TGF-β blocker treated mature adipocytes had considerably higher levels of glycerol and triglycerides than the control group, whereas GLUTs and lipases expression levels were unchanged. These findings show that inhibiting the abnormally upregulated TGF-β pathway will only restore iWAT expansion and ameliorate the global metabolic malfunction of glucose and lipids in lipodystrophy, not obesity.
Keywords: TGF-β; adipose atrophy; adipose hypertrophy; adipose tissue; glucolipid metabolism.
© 2024 The Authors. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Transgenic Mice Overexpressing SREBP-1a in Male ob/ob Mice Exhibit Lipodystrophy and Exacerbate Insulin Resistance.Endocrinology. 2018 Jun 1;159(6):2308-2323. doi: 10.1210/en.2017-03179. Endocrinology. 2018. PMID: 29668871
-
Overexpression of sterol regulatory element-binding protein-1a in mouse adipose tissue produces adipocyte hypertrophy, increased fatty acid secretion, and fatty liver.J Biol Chem. 2003 Sep 19;278(38):36652-60. doi: 10.1074/jbc.M306540200. Epub 2003 Jul 10. J Biol Chem. 2003. PMID: 12855691
-
Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus.J Biol Chem. 1999 Oct 15;274(42):30028-32. doi: 10.1074/jbc.274.42.30028. J Biol Chem. 1999. PMID: 10514488
-
Anti-obesity effect of a standardised ethanol extract from Curcuma longa L. fermented with Aspergillus oryzae in ob/ob mice and primary mouse adipocytes.J Sci Food Agric. 2012 Jul;92(9):1833-40. doi: 10.1002/jsfa.5592. Epub 2012 Jan 25. J Sci Food Agric. 2012. PMID: 22278718
-
Ectopic lipid accumulation: A potential cause for metabolic disturbances and a contributor to the alteration of kidney function.Biochimie. 2013 Nov;95(11):1971-9. doi: 10.1016/j.biochi.2013.07.017. Epub 2013 Jul 27. Biochimie. 2013. PMID: 23896376 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

