Design, synthesis, pharmacological evaluation, and in silico studies of the activity of novel spiro pyrrolo[3,4- d]pyrimidine derivatives
- PMID: 38174254
- PMCID: PMC10759174
- DOI: 10.1039/d3ra07078f
Design, synthesis, pharmacological evaluation, and in silico studies of the activity of novel spiro pyrrolo[3,4- d]pyrimidine derivatives
Erratum in
-
Correction: Design, synthesis, pharmacological evaluation, and in silico studies of the activity of novel spiro pyrrolo[3,4-d]pyrimidine derivatives.RSC Adv. 2024 Nov 13;14(49):36351. doi: 10.1039/d4ra90128b. eCollection 2024 Nov 11. RSC Adv. 2024. PMID: 39539533 Free PMC article.
Abstract
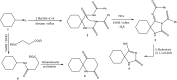
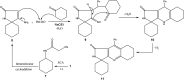
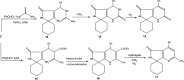
In the present study, spiro compounds are shown to have distinctive characteristics because of their interesting conformations and their structural impacts on biological systems. A new family of functionalized spiro pyrrolo[3,4-d]pyrimidines is prepared via the one-pot condensation reaction of amino cyclohexane derivatives with benzaldehyde to prepare fused azaspiroundecanedione and azaspirodecenone/thione derivatives. A series of synthesized spiro compounds were scanned against DPPH and evaluated for their ability to inhibit COX-1 and COX-2. All compounds exhibit significant antiinflammatory activity, and they inhibited both COX-1 and COX-2 enzymes with a selectivity index higher than celecoxib as a reference drug. The most powerful and selective COX-2 inhibitor compounds were 11 and 6, with selectivity indices of 175 and 129.21 in comparison to 31.52 of the standard celecoxib. However, candidate 14 showed a very promising antiinflammatory activity with an IC50 of 6.00, while celecoxib had an IC50 of 14.50. Our findings are promising in the area of medicinal chemistry for further optimization of the newly designed and synthesized compounds regarding the discussed structure-activity relationship study (SAR), in order to obtain a superior antioxidant lead compound in the near future. All chemical structures of the novel synthesized candidates were unequivocally elucidated and confirmed utilizing spectroscopic and elemental investigations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Molecular Dynamics and Biological Evaluation of 2-chloro-7-cyclopentyl- 7H-pyrrolo[2,3-d]pyrimidine Derivatives Against Breast Cancer.Comb Chem High Throughput Screen. 2017;20(8):703-712. doi: 10.2174/1386207320666170724110015. Comb Chem High Throughput Screen. 2017. PMID: 28738766
-
Design, synthesis, biological evaluation and in silico studies of novel pyrrolo[3,4-d]pyridazinone derivatives withpromising anti-inflammatory and antioxidant activity.Bioorg Chem. 2020 Sep;102:104035. doi: 10.1016/j.bioorg.2020.104035. Epub 2020 Jun 19. Bioorg Chem. 2020. PMID: 32721780
-
Design and Synthesis of Novel 1,3,4-Oxadiazole and 1,2,4-Triazole Derivatives as Cyclooxygenase-2 Inhibitors with Anti-inflammatory and Antioxidant activity in LPS-stimulated RAW264.7 Macrophages.Bioorg Chem. 2022 Jul;124:105808. doi: 10.1016/j.bioorg.2022.105808. Epub 2022 Apr 13. Bioorg Chem. 2022. PMID: 35447409 Free PMC article.
-
Therapeutic potential of anticancer activity of nitrogen-containing heterocyclic scaffolds as Janus kinase (JAK) inhibitor: Biological activity, selectivity, and structure-activity relationship.Bioorg Chem. 2024 Nov;152:107696. doi: 10.1016/j.bioorg.2024.107696. Epub 2024 Aug 8. Bioorg Chem. 2024. PMID: 39167870 Review.
-
The Application of Pyrrolo[2, 3-d]pyrimidine Scaffold in Medicinal Chemistry from 2017 to 2021.Mini Rev Med Chem. 2023;23(10):1118-1136. doi: 10.2174/1389557523666230111161810. Mini Rev Med Chem. 2023. PMID: 36635929 Review.
Cited by
-
Pyrimidines: A New Versatile Molecule in the Drug Development Field, Scope, and Future Aspects.Pharmaceuticals (Basel). 2024 Sep 24;17(10):1258. doi: 10.3390/ph17101258. Pharmaceuticals (Basel). 2024. PMID: 39458899 Free PMC article. Review.
References
-
- Moghaddam-Manesh M. Ghazanfari D. Sheikhhosseini E. Akhgar M. MgO-Nanoparticle-Catalyzed Synthesis and Evaluation of Antimicrobial and Antioxidant Activity of New Multi-Ring Compounds Containing Spiro[indoline-3,4′-[1,3] dithiine] ChemistrySelect. 2019;4(31):9247–9251. doi: 10.1002/slct.201900935. doi: 10.1002/slct.201900935. - DOI - DOI
-
- Balasubramanian S. Ramalingan C. Aridoss G. Kabilan S. Synthesis, and study of antibacterial and antifungal activities of novel 8-methyl-7,9-diaryl1,2,4,8-tetraazaspiro [4.5] decan-3-thiones. Eur. J. Med. Chem. 2005;40:694–700. doi: 10.1016/j.ejmech.2005.02.001. doi: 10.1016/j.ejmech.2005.02.001. - DOI - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous