Development and Clinical Applications of PI3K/AKT/mTOR Pathway Inhibitors as a Therapeutic Option for Leukemias
- PMID: 38173664
- PMCID: PMC10758851
- DOI: 10.21873/cdp.10279
Development and Clinical Applications of PI3K/AKT/mTOR Pathway Inhibitors as a Therapeutic Option for Leukemias
Abstract
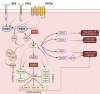
Leukemias are hematological neoplasms characterized by dysregulations in several cellular signaling pathways, prominently including the PI3K/AKT/mTOR pathway. Since this pathway is associated with several important cellular mechanisms, such as proliferation, metabolism, survival, and cell death, its hyperactivation significantly contributes to the development of leukemias. In addition, it is a crucial prognostic factor, often correlated with therapeutic resistance. Changes in the PI3K/AKT/mTOR pathway are identified in more than 50% of cases of acute leukemia, especially in myeloid lineages. Furthermore, these changes are highly frequent in cases of chronic lymphocytic leukemia, especially those with a B cell phenotype, due to the correlation between the hyperactivation of B cell receptors and the abnormal activation of PI3Kδ. Thus, the search for new therapies that inhibit the activity of the PI3K/AKT/mTOR pathway has become the objective of several clinical studies that aim to replace conventional oncological treatments that have high rates of toxicities and low specificity with target-specific therapies offering improved patient quality of life. In this review we describe the PI3K/AKT/mTOR signal transduction pathway and its implications in leukemogenesis. Furthermore, we provide an overview of clinical trials that employed PI3K/AKT/mTOR inhibitors either as monotherapy or in combination with other cytotoxic agents for treating patients with various types of leukemias. The varying degrees of treatment efficacy are also reported.
Keywords: Leukemia; PI3K/AKT/mTOR; kinase inhibitors; molecular biomarkers; review; target therapy.
Copyright 2024, International Institute of Anticancer Research.
Conflict of interest statement
The Authors declare no conflict of interest in relation to this study. The funders had no role in the design of the study; in the collection, analyses, or data interpretation; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer.Semin Cancer Biol. 2022 Oct;85:69-94. doi: 10.1016/j.semcancer.2021.06.019. Epub 2021 Jun 25. Semin Cancer Biol. 2022. PMID: 34175443 Review.
-
Synergistic effects of selective inhibitors targeting the PI3K/AKT/mTOR pathway or NUP214-ABL1 fusion protein in human Acute Lymphoblastic Leukemia.Oncotarget. 2016 Nov 29;7(48):79842-79853. doi: 10.18632/oncotarget.13035. Oncotarget. 2016. PMID: 27821800 Free PMC article.
-
Targeting the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway: an emerging treatment strategy for squamous cell lung carcinoma.Cancer Treat Rev. 2014 Sep;40(8):980-9. doi: 10.1016/j.ctrv.2014.06.006. Epub 2014 Jul 3. Cancer Treat Rev. 2014. PMID: 25037117 Review.
-
PI3K/Akt/mTOR Signaling Pathway in Blood Malignancies-New Therapeutic Possibilities.Cancers (Basel). 2023 Nov 5;15(21):5297. doi: 10.3390/cancers15215297. Cancers (Basel). 2023. PMID: 37958470 Free PMC article. Review.
-
Two hits are better than one: targeting both phosphatidylinositol 3-kinase and mammalian target of rapamycin as a therapeutic strategy for acute leukemia treatment.Oncotarget. 2012 Apr;3(4):371-94. doi: 10.18632/oncotarget.477. Oncotarget. 2012. PMID: 22564882 Free PMC article. Review.
Cited by
-
Monocytic Differentiation in Acute Myeloid Leukemia Cells: Diagnostic Criteria, Biological Heterogeneity, Mitochondrial Metabolism, Resistance to and Induction by Targeted Therapies.Int J Mol Sci. 2024 Jun 8;25(12):6356. doi: 10.3390/ijms25126356. Int J Mol Sci. 2024. PMID: 38928061 Free PMC article. Review.
References
-
- Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, Chen W, Chen X, Chng WJ, Choi JK, Colmenero I, Coupland SE, Cross NCP, De Jong D, Elghetany MT, Takahashi E, Emile JF, Ferry J, Fogelstrand L, Fontenay M, Germing U, Gujral S, Haferlach T, Harrison C, Hodge JC, Hu S, Jansen JH, Kanagal-Shamanna R, Kantarjian HM, Kratz CP, Li XQ, Lim MS, Loeb K, Loghavi S, Marcogliese A, Meshinchi S, Michaels P, Naresh KN, Natkunam Y, Nejati R, Ott G, Padron E, Patel KP, Patkar N, Picarsic J, Platzbecker U, Roberts I, Schuh A, Sewell W, Siebert R, Tembhare P, Tyner J, Verstovsek S, Wang W, Wood B, Xiao W, Yeung C, Hochhaus A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and histiocytic/dendriticneoplasms. Leukemia. 2022;36(7):1703–1719. doi: 10.1038/s41375-022-01613-1. - DOI - PMC - PubMed
-
- Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid neoplasms. Leukemia. 2022;36(7):1720–1748. doi: 10.1038/s41375-022-01620-2. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
