A systematic review for the development of Alzheimer's disease in in vitro models: a focus on different inducing agents
- PMID: 38173557
- PMCID: PMC10761490
- DOI: 10.3389/fnagi.2023.1296919
A systematic review for the development of Alzheimer's disease in in vitro models: a focus on different inducing agents
Abstract
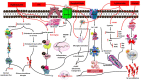
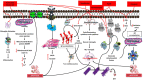
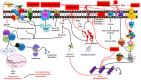
Alzheimer's disease (AD) is the most common progressive neurodegenerative disease and is associated with dementia. Presently, various chemical and environmental agents are used to induce in-vitro models of Alzheimer disease to investigate the efficacy of different therapeutic drugs. We screened literature from databases such as PubMed, ScienceDirect, and Google scholar, emphasizing the diverse targeting mechanisms of neuro degeneration explored in in-vitro models. The results revealed studies in which different types of chemicals and environmental agents were used for in-vitro development of Alzheimer-targeting mechanisms of neurodegeneration. Studies using chemically induced in-vitro AD models included in this systematic review will contribute to a deeper understanding of AD. However, none of these models can reproduce all the characteristics of disease progression seen in the majority of Alzheimer's disease subtypes. Additional modifications would be required to replicate the complex conditions of human AD in an exact manner. In-vitro models of Alzheimer's disease developed using chemicals and environmental agents are instrumental in providing insights into the disease's pathophysiology; therefore, chemical-induced in-vitro AD models will continue to play vital role in future AD research. This systematic screening revealed the pivotal role of chemical-induced in-vitro AD models in advancing our understanding of AD pathophysiology and is therefore important to understand the potential of these chemicals in AD pathogenesis.
Keywords: Alzheimer’s disease; amyloid beta; in-vitro models; neurodegeneration; tau protein.
Copyright © 2023 Prajapat, Kaur, Choudhary, Pahwa, Bansal, Joshi, Batra, Mishra, Singla, Kaur, Prabha, Patel and Medhi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Research on Alzheimer's Disease (AD) Involving the Use of In vivo and In vitro Models and Mechanisms.Cent Nerv Syst Agents Med Chem. 2024 May 27. doi: 10.2174/0118715249293642240522054929. Online ahead of print. Cent Nerv Syst Agents Med Chem. 2024. PMID: 38803173
-
Insights into the Pathophysiology of Alzheimer's Disease and Potential Therapeutic Targets: A Current Perspective.J Alzheimers Dis. 2023;91(2):507-530. doi: 10.3233/JAD-220666. J Alzheimers Dis. 2023. PMID: 36502321 Review.
-
Potential Neuroprotective Strategies using Smart Drug Delivery Systems for Alzheimer's Disease.Infect Disord Drug Targets. 2024;24(3):e231023222565. doi: 10.2174/0118715265254985231012065058. Infect Disord Drug Targets. 2024. PMID: 37873911 Review.
-
Inverse relationship between Alzheimer's disease and cancer, and other factors contributing to Alzheimer's disease: a systematic review.BMC Neurol. 2016 Nov 22;16(1):236. doi: 10.1186/s12883-016-0765-2. BMC Neurol. 2016. PMID: 27875990 Free PMC article. Review.
-
Carthamus tinctorius L.: A natural neuroprotective source for anti-Alzheimer's disease drugs.J Ethnopharmacol. 2022 Nov 15;298:115656. doi: 10.1016/j.jep.2022.115656. Epub 2022 Aug 27. J Ethnopharmacol. 2022. PMID: 36041691 Review.
References
-
- Atzori C., Ghetti B., Piva R., Srinivasan A. N., Zolo P., Delisle M. B., et al. . (2001). Activation of the JNK/p 38 pathway occurs in diseases characterized by tau protein pathology and is related to tau phosphorylation but not to apoptosis. J. Neuropathol. Exp. Neurol. 60, 1190–1197. doi: 10.1093/jnen/60.12.1190, PMID: - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

