Lymphatic vessel: origin, heterogeneity, biological functions, and therapeutic targets
- PMID: 38172098
- PMCID: PMC10764842
- DOI: 10.1038/s41392-023-01723-x
Lymphatic vessel: origin, heterogeneity, biological functions, and therapeutic targets
Abstract
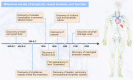
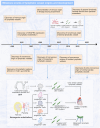
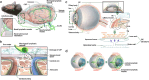
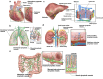
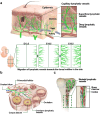
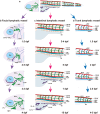
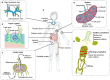
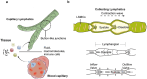
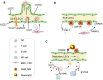
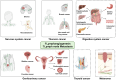
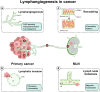
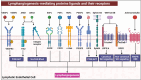
Lymphatic vessels, comprising the secondary circulatory system in human body, play a multifaceted role in maintaining homeostasis among various tissues and organs. They are tasked with a serious of responsibilities, including the regulation of lymph absorption and transport, the orchestration of immune surveillance and responses. Lymphatic vessel development undergoes a series of sophisticated regulatory signaling pathways governing heterogeneous-origin cell populations stepwise to assemble into the highly specialized lymphatic vessel networks. Lymphangiogenesis, as defined by new lymphatic vessels sprouting from preexisting lymphatic vessels/embryonic veins, is the main developmental mechanism underlying the formation and expansion of lymphatic vessel networks in an embryo. However, abnormal lymphangiogenesis could be observed in many pathological conditions and has a close relationship with the development and progression of various diseases. Mechanistic studies have revealed a set of lymphangiogenic factors and cascades that may serve as the potential targets for regulating abnormal lymphangiogenesis, to further modulate the progression of diseases. Actually, an increasing number of clinical trials have demonstrated the promising interventions and showed the feasibility of currently available treatments for future clinical translation. Targeting lymphangiogenic promoters or inhibitors not only directly regulates abnormal lymphangiogenesis, but improves the efficacy of diverse treatments. In conclusion, we present a comprehensive overview of lymphatic vessel development and physiological functions, and describe the critical involvement of abnormal lymphangiogenesis in multiple diseases. Moreover, we summarize the targeting therapeutic values of abnormal lymphangiogenesis, providing novel perspectives for treatment strategy of multiple human diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
IGFBP7 functions as a potential lymphangiogenesis inducer in non-small cell lung carcinoma.Oncol Rep. 2016 Mar;35(3):1483-92. doi: 10.3892/or.2015.4516. Epub 2015 Dec 24. Oncol Rep. 2016. PMID: 26706909
-
Digging deeper into lymphatic vessel formation in vitro and in vivo.BMC Cell Biol. 2011 Jun 24;12:29. doi: 10.1186/1471-2121-12-29. BMC Cell Biol. 2011. PMID: 21702933 Free PMC article.
-
Sonic hedgehog selectively promotes lymphangiogenesis after kidney injury through noncanonical pathway.Am J Physiol Renal Physiol. 2019 Oct 1;317(4):F1022-F1033. doi: 10.1152/ajprenal.00077.2019. Epub 2019 Aug 14. Am J Physiol Renal Physiol. 2019. PMID: 31411078 Free PMC article.
-
Impact of Hemostasis on the Lymphatic System in Development and Disease.Arterioscler Thromb Vasc Biol. 2023 Oct;43(10):1747-1754. doi: 10.1161/ATVBAHA.123.318824. Epub 2023 Aug 3. Arterioscler Thromb Vasc Biol. 2023. PMID: 37534465 Review.
-
Biomaterial Based Strategies for Engineering New Lymphatic Vasculature.Adv Healthc Mater. 2020 Sep;9(18):e2000895. doi: 10.1002/adhm.202000895. Epub 2020 Jul 30. Adv Healthc Mater. 2020. PMID: 32734721 Free PMC article. Review.
Cited by
-
Tumor-associated lymphatic vessel density is a reliable biomarker for prognosis of esophageal cancer after radical resection: a systemic review and meta-analysis.Front Immunol. 2024 Sep 20;15:1453482. doi: 10.3389/fimmu.2024.1453482. eCollection 2024. Front Immunol. 2024. PMID: 39372418 Free PMC article.
-
The role of lymphatic endothelial cell metabolism in lymphangiogenesis and disease.Front Cardiovasc Med. 2024 May 9;11:1392816. doi: 10.3389/fcvm.2024.1392816. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38798921 Free PMC article. Review.
-
Advances in lymphatic metastasis of non-small cell lung cancer.Cell Commun Signal. 2024 Apr 2;22(1):201. doi: 10.1186/s12964-024-01574-1. Cell Commun Signal. 2024. PMID: 38566083 Free PMC article. Review.
-
Mechanisms of Myocardial Edema Development in CVD Pathophysiology.Biomedicines. 2024 Feb 19;12(2):465. doi: 10.3390/biomedicines12020465. Biomedicines. 2024. PMID: 38398066 Free PMC article. Review.
-
Hyaluronic acid modified indocyanine green nanoparticles: a novel targeted strategy for NIR-II fluorescence lymphatic imaging.Front Chem. 2024 Jul 3;12:1435627. doi: 10.3389/fchem.2024.1435627. eCollection 2024. Front Chem. 2024. PMID: 39021390 Free PMC article.
References
-
- Irschick, R., Siemon, C. & Brenner, E. The history of anatomical research of lymphatics—from the ancient times to the end of the European Renaissance. Ann. Anat.223, 49–69 (2019). - PubMed
-
- Kanter, M. A. The lymphatic system: an historical perspective. Plast. Reconstr. Surg.79, 131–139 (1987). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

