The NFκB signaling system in the generation of B-cell subsets: from germinal center B cells to memory B cells and plasma cells
- PMID: 38169968
- PMCID: PMC10758606
- DOI: 10.3389/fimmu.2023.1185597
The NFκB signaling system in the generation of B-cell subsets: from germinal center B cells to memory B cells and plasma cells
Abstract
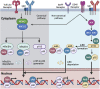
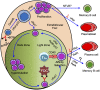
Memory B cells and antibody-secreting cells are the two prime effector B cell populations that drive infection- and vaccine-induced long-term antibody-mediated immunity. The antibody-mediated immunity mostly relies on the formation of specialized structures within secondary lymphoid organs, called germinal centers (GCs), that facilitate the interactions between B cells, T cells, and antigen-presenting cells. Antigen-activated B cells may proliferate and differentiate into GC-independent plasmablasts and memory B cells or differentiate into GC B cells. The GC B cells undergo proliferation coupled to somatic hypermutation of their immunoglobulin genes for antibody affinity maturation. Subsequently, affinity mature GC B cells differentiate into GC-dependent plasma cells and memory B cells. Here, we review how the NFκB signaling system controls B cell proliferation and the generation of GC B cells, plasmablasts/plasma cells, and memory B cells. We also identify and discuss some important unanswered questions in this connection.
Keywords: B cell; NFκB; and cell signaling; memory B cell; plasma cell.
Copyright © 2023 Roy, Chakraborty, Kumar, Manna and Roy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Tracking Immunoglobulin Repertoire and Transcriptomic Changes in Germinal Center B Cells by Single-Cell Analysis.Front Immunol. 2022 Jan 12;12:818758. doi: 10.3389/fimmu.2021.818758. eCollection 2021. Front Immunol. 2022. PMID: 35095922 Free PMC article.
-
Germinal Centre Shutdown.Front Immunol. 2021 Jul 7;12:705240. doi: 10.3389/fimmu.2021.705240. eCollection 2021. Front Immunol. 2021. PMID: 34305944 Free PMC article. Review.
-
Germinal Center and Extrafollicular B Cell Responses in Vaccination, Immunity, and Autoimmunity.Immunity. 2020 Dec 15;53(6):1136-1150. doi: 10.1016/j.immuni.2020.11.006. Immunity. 2020. PMID: 33326765 Free PMC article. Review.
-
Plasma cell differentiation during the germinal center reaction.Immunol Rev. 2019 Mar;288(1):64-74. doi: 10.1111/imr.12751. Immunol Rev. 2019. PMID: 30874351 Review.
-
Early T Follicular Helper Cell Responses and Germinal Center Reactions Are Associated with Viremia Control in Immunized Rhesus Macaques.J Virol. 2019 Feb 5;93(4):e01687-18. doi: 10.1128/JVI.01687-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463978 Free PMC article.
Cited by
-
Transcriptome Profiling Associated with CARD11 Overexpression in Colorectal Cancer Implicates a Potential Role for Tumor Immune Microenvironment and Cancer Pathways Modulation via NF-κB.Int J Mol Sci. 2024 Sep 26;25(19):10367. doi: 10.3390/ijms251910367. Int J Mol Sci. 2024. PMID: 39408697 Free PMC article.
-
Direct observation correlates NFκB cRel in B cells with activating and terminating their proliferative program.Proc Natl Acad Sci U S A. 2024 Jul 23;121(30):e2309686121. doi: 10.1073/pnas.2309686121. Epub 2024 Jul 18. Proc Natl Acad Sci U S A. 2024. PMID: 39024115 Free PMC article.
References
-
- Nakagawa R, Toboso-Navasa A, Schips M, Young G, Bhaw-Rosun L, Llorian-Sopena M, et al. . Permissive selection followed by affinity-based proliferation of GC light zone B cells dictates cell fate and ensures clonal breadth. Proc Natl Acad Sci USA (2021) 118:e2016425118. doi: 10.1073/pnas.2016425118 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

