Therapeutic targeting of p90 ribosomal S6 kinase
- PMID: 38169775
- PMCID: PMC10758423
- DOI: 10.3389/fcell.2023.1297292
Therapeutic targeting of p90 ribosomal S6 kinase
Abstract
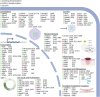
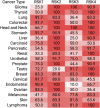
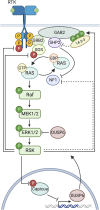
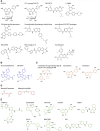
The Serine/Threonine protein kinase family, p90 ribosomal S6 kinases (RSK) are downstream effectors of extracellular signal regulated kinase 1/2 (ERK1/2) and are activated in response to tyrosine kinase receptor or G-protein coupled receptor signaling. RSK contains two distinct kinase domains, an N-terminal kinase (NTKD) and a C-terminal kinase (CTKD). The sole function of the CTKD is to aid in the activation of the NTKD, which is responsible for substrate phosphorylation. RSK regulates various homeostatic processes including those involved in transcription, translation and ribosome biogenesis, proliferation and survival, cytoskeleton, nutrient sensing, excitation and inflammation. RSK also acts as a major negative regulator of ERK1/2 signaling. RSK is associated with numerous cancers and has been primarily studied in the context of transformation and metastasis. The development of specific RSK inhibitors as cancer therapeutics has lagged behind that of other members of the mitogen-activated protein kinase signaling pathway. Importantly, a pan-RSK inhibitor, PMD-026, is currently in phase I/1b clinical trials for metastatic breast cancer. However, there are four members of the RSK family, which have overlapping and distinct functions that can vary in a tissue specific manner. Thus, a problem for transitioning a RSK inhibitor to the clinic may be the necessity to develop isoform specific inhibitors, which will be challenging as the NTKDs are very similar to each other. CTKD inhibitors have limited use as therapeutics as they are not able to inhibit the activity of the NTKD but could be used in the development of proteolysis-targeting chimeras.
Keywords: RSK; p90 ribosomal S6 kinase; p90RSK; phosphorylation; small molecule inhibitor; substrate.
Copyright © 2023 Wright and Lannigan.
Conflict of interest statement
DL holds patents on the RSK analogues based on SL0101. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates extracellular signal-regulated kinase docking and RSK activity.Mol Cell Biol. 2003 Jul;23(14):4796-804. doi: 10.1128/MCB.23.14.4796-4804.2003. Mol Cell Biol. 2003. PMID: 12832467 Free PMC article.
-
Activation and inhibition of the C-terminal kinase domain of p90 ribosomal S6 kinases.Life Sci Alliance. 2023 Feb 20;6(5):e202201425. doi: 10.26508/lsa.202201425. Print 2023 May. Life Sci Alliance. 2023. PMID: 36806093 Free PMC article.
-
Characterization of regulatory events associated with membrane targeting of p90 ribosomal S6 kinase 1.Mol Cell Biol. 2001 Nov;21(21):7470-80. doi: 10.1128/MCB.21.21.7470-7480.2001. Mol Cell Biol. 2001. PMID: 11585927 Free PMC article.
-
Ribosomal S6 kinase (RSK) modulators: a patent review.Expert Opin Ther Pat. 2016 Sep;26(9):1061-78. doi: 10.1080/13543776.2016.1212839. Epub 2016 Aug 1. Expert Opin Ther Pat. 2016. PMID: 27410995 Review.
-
The RSK factors of activating the Ras/MAPK signaling cascade.Front Biosci. 2008 May 1;13:4258-75. doi: 10.2741/3003. Front Biosci. 2008. PMID: 18508509 Review.
Cited by
-
Adaptive protein synthesis in genetic models of copper deficiency and childhood neurodegeneration.bioRxiv [Preprint]. 2024 Nov 18:2024.09.09.612106. doi: 10.1101/2024.09.09.612106. bioRxiv. 2024. Update in: Mol Biol Cell. 2025 Mar 1;36(3):ar33. doi: 10.1091/mbc.E24-11-0512. PMID: 39314281 Free PMC article. Updated. Preprint.
-
Targeting the p90RSK/MDM2/p53 Pathway Is Effective in Blocking Tumors with Oncogenic Up-Regulation of the MAPK Pathway Such as Melanoma and Lung Cancer.Cells. 2024 Sep 14;13(18):1546. doi: 10.3390/cells13181546. Cells. 2024. PMID: 39329730 Free PMC article.
-
Ribosomal S6 kinase (RSK) plays a critical role in DNA damage response via the phosphorylation of histone lysine demethylase KDM4B.Breast Cancer Res. 2024 Oct 21;26(1):146. doi: 10.1186/s13058-024-01901-x. Breast Cancer Res. 2024. PMID: 39434131 Free PMC article.
References
-
- Arechavaleta-Velasco F., Zeferino-Toquero M., Estrada-Moscoso I., Imani-Razavi F. S., Olivares A., Perez-Juarez C. E., et al. (2016). Ribosomal S6 kinase 4 (RSK4) expression in ovarian tumors and its regulation by antineoplastic drugs in ovarian cancer cell lines. Med. Oncol. 33, 11. 10.1007/s12032-015-0724-6 - DOI - PubMed
-
- Aronchik I., Appleton B. A., Basham S. E., Crawford K., Del Rosario M., Doyle L. V., et al. (2014). Novel potent and selective inhibitors of p90 ribosomal S6 kinase reveal the heterogeneity of RSK function in MAPK-driven cancers. Mol. Cancer Res. 12, 803–812. 10.1158/1541-7786.MCR-13-0595 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

