ZIKV Inhibitors Based on Pyrazolo[3,4- d]pyridazine-7-one Core: Rational Design, In Vitro Evaluation, and Theoretical Studies
- PMID: 38162759
- PMCID: PMC10753549
- DOI: 10.1021/acsomega.3c06612
ZIKV Inhibitors Based on Pyrazolo[3,4- d]pyridazine-7-one Core: Rational Design, In Vitro Evaluation, and Theoretical Studies
Abstract
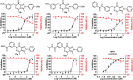
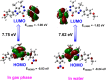
The Zika virus (ZIKV) is believed to cause birth defects, and no anti-ZIKV drugs have been approved by medical organizations to date. Starting from antimicrobial lead compounds with a pyrazolo[3,4-d]pyridazine-7-one scaffold, we synthesized 16 derivatives and screened their ability to interfere with ZIKV infection utilizing a cell-based phenotypic assay. Of these, five compounds showed significant inhibition of ZIKV with a selective index value greater than 4.6. In particular, compound 9b showed the best anti-ZIKV activity with a selectivity index of 22.4 (half-maximal effective concentration = 25.6 μM and 50% cytotoxic concentration = 572.4 μM). Through the brine shrimp lethality bioassay, 9b, 10b, 12, 17a, and 19a showed median lethal dose values in a range of 87.2-100.3 μg/mL. Compound 9b was also targeted to the NS2B-NS3 protease of ZIKV using molecular docking protocols, in which it acted as a noncompetitive inhibitor and strongly bound to five key amino acids (His51, Asp75, Ser135, Ala132, Tyr161). Utilizing the pharmacophore model of 9b, the top 20 hits were identified as prospective inhibitors of NS2B-NS3 protease, and six of them were confirmed for their stability with the protease via redocking and molecular dynamics simulations.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Development of NS2B-NS3 protease inhibitor that impairs Zika virus replication.Virus Res. 2023 May;329:199092. doi: 10.1016/j.virusres.2023.199092. Epub 2023 Apr 5. Virus Res. 2023. PMID: 36965673 Free PMC article.
-
Identification of a C2-symmetric diol based human immunodeficiency virus protease inhibitor targeting Zika virus NS2B-NS3 protease.J Biomol Struct Dyn. 2020 Nov;38(18):5526-5536. doi: 10.1080/07391102.2019.1704882. Epub 2019 Dec 27. J Biomol Struct Dyn. 2020. PMID: 31880199
-
NS2B-NS3 protease inhibitors as promising compounds in the development of antivirals against Zika virus: A systematic review.J Med Virol. 2022 Feb;94(2):442-453. doi: 10.1002/jmv.27386. Epub 2021 Oct 20. J Med Virol. 2022. PMID: 34636434
-
Advances in Computational Methods to Discover New NS2B-NS3 Inhibitors Useful Against Dengue and Zika Viruses.Curr Top Med Chem. 2022;22(29):2435-2462. doi: 10.2174/1568026623666221122121330. Curr Top Med Chem. 2022. PMID: 36415099 Review.
-
The Structure of the Zika Virus Protease, NS2B/NS3pro.Adv Exp Med Biol. 2018;1062:131-145. doi: 10.1007/978-981-10-8727-1_10. Adv Exp Med Biol. 2018. PMID: 29845530 Review.
Cited by
-
Discovery of a novel benzimidazole conjugated quinazolinone derivative as a promising SARS-CoV-2 3CL protease inhibitor.RSC Adv. 2024 Oct 24;14(46):33820-33829. doi: 10.1039/d4ra03267e. eCollection 2024 Oct 23. RSC Adv. 2024. PMID: 39450066 Free PMC article.
References
-
- Nguyen C. Q.; Nguyen T. H. M.; Nguyen T. T. T.; Bui T. B. H.; Nguyen T. T.; Huynh N. T.; Le T. D.; Nguyen T. M. P.; Nguyen D. T.; Nguyen M. T.; Pham M. Q.; Tran Q. D.; Nguyen H. P. Designs, synthesis, docking studies, and biological evaluation of novel berberine derivatives targeting zika virus. J. Chem. 2021, 2021, e556711110.1155/2021/5567111. - DOI
LinkOut - more resources
Full Text Sources

