SARS-CoV-2 decreases malaria severity in co-infected rodent models
- PMID: 38156320
- PMCID: PMC10753813
- DOI: 10.3389/fcimb.2023.1307553
SARS-CoV-2 decreases malaria severity in co-infected rodent models
Abstract
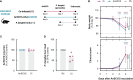
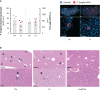
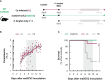
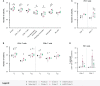
Coronavirus disease 2019 (COVID-19) and malaria, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Plasmodium parasites, respectively, share geographical distribution in regions where the latter disease is endemic, leading to the emergence of co-infections between the two pathogens. Thus far, epidemiologic studies and case reports have yielded insufficient data on the reciprocal impact of the two pathogens on either infection and related diseases. We established novel co-infection models to address this issue experimentally, employing either human angiotensin-converting enzyme 2 (hACE2)-expressing or wild-type mice, in combination with human- or mouse-infective variants of SARS-CoV-2, and the P. berghei rodent malaria parasite. We now show that a primary infection by a viral variant that causes a severe disease phenotype partially impairs a subsequent liver infection by the malaria parasite. Additionally, exposure to an attenuated viral variant modulates subsequent immune responses and provides protection from severe malaria-associated outcomes when a blood stage P. berghei infection was established. Our findings unveil a hitherto unknown host-mediated virus-parasite interaction that could have relevant implications for disease management and control in malaria-endemic regions. This work may contribute to the development of other models of concomitant infection between Plasmodium and respiratory viruses, expediting further research on co-infections that lead to complex disease presentations.
Keywords: COVID-19; Plasmodium; SARS-CoV-2; co-infection; malaria.
Copyright © 2023 Fraga, Mósca, Moita, Simas, Nunes-Cabaço and Prudêncio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Hamsters Expressing Human Angiotensin-Converting Enzyme 2 Develop Severe Disease following Exposure to SARS-CoV-2.mBio. 2022 Feb 22;13(1):e0290621. doi: 10.1128/mbio.02906-21. Epub 2022 Jan 25. mBio. 2022. PMID: 35073750 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
SARS-CoV-2 Causes Lung Infection without Severe Disease in Human ACE2 Knock-In Mice.J Virol. 2022 Jan 12;96(1):e0151121. doi: 10.1128/JVI.01511-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34668780 Free PMC article.
-
The origins, isolation, and biological characterization of rodent malaria parasites.Parasitol Int. 2022 Dec;91:102636. doi: 10.1016/j.parint.2022.102636. Epub 2022 Aug 1. Parasitol Int. 2022. PMID: 35926694 Free PMC article. Review.
-
K18- and CAG-hACE2 Transgenic Mouse Models and SARS-CoV-2: Implications for Neurodegeneration Research.Molecules. 2022 Jun 28;27(13):4142. doi: 10.3390/molecules27134142. Molecules. 2022. PMID: 35807384 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

