Combining adhesive and nonadhesive injectable hydrogels for intervertebral disc repair in an ovine discectomy model
- PMID: 38156055
- PMCID: PMC10751969
- DOI: 10.1002/jsp2.1293
Combining adhesive and nonadhesive injectable hydrogels for intervertebral disc repair in an ovine discectomy model
Abstract
Background: Intervertebral disc (IVD) disorders (e.g., herniation) directly contribute to back pain, which is a leading cause of global disability. Next-generation treatments for IVD herniation need advanced preclinical testing to evaluate their ability to repair large defects, prevent reherniation, and limit progressive degeneration. This study tested whether experimental, injectable, and nonbioactive biomaterials could slow IVD degeneration in an ovine discectomy model.
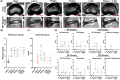
Methods: Ten skeletally mature sheep (4-5.5 years) experienced partial discectomy injury with cruciate-style annulus fibrosus (AF) defects and 0.1 g nucleus pulposus (NP) removal in the L1-L2, L2-L3, and L3-L4 lumbar IVDs. L4-L5 IVDs were Intact controls. IVD injury levels received: (1) no treatment (Injury), (2) poly (ethylene glycol) diacrylate (PEGDA), (3) genipin-crosslinked fibrin (FibGen), (4) carboxymethylcellulose-methylcellulose (C-MC), or (5) C-MC and FibGen (FibGen + C-MC). Animals healed for 12 weeks, then IVDs were assessed using computed tomography (CT), magnetic resonance (MR) imaging, and histopathology.
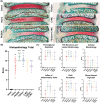
Results: All repaired IVDs retained ~90% of their preoperative disc height and showed minor degenerative changes by Pfirrmann grading. All repairs had similar disc height loss and Pfirrmann grade as Injury IVDs. Adhesive AF sealants (i.e., PEGDA and FibGen) did not herniate, although repair caused local endplate (EP) changes and inflammation. NP repair biomaterials (i.e., C-MC) and combination repair (i.e., FibGen + C-MC) exhibited lower levels of degeneration, less EP damage, and less severe inflammation; however, C-MC showed signs of herniation via biomaterial expulsion.
Conclusions: All repair IVDs were noninferior to Injury IVDs by IVD height loss and Pfirrmann grade. C-MC and FibGen + C-MC IVDs had the best outcomes, and may be appropriate for enhancement with bioactive factors (e.g., cells, growth factors, and miRNAs). Such bioactive factors appear to be necessary to prevent injury-induced IVD degeneration. Application of AF sealants alone (i.e., PEGDA and FibGen) resulted in EP damage and inflammation, particularly for PEGDA IVDs, suggesting further material refinements are needed.
Keywords: annulus fibrosus repair; carboxymethylcellulose–methylcellulose; fibrin; hydrogels; injectable biomaterials; intervertebral disc; nucleus pulposus repair; ovine discectomy model; poly(ethylene glycol) diacrylate.
© 2023 The Authors. JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Figures
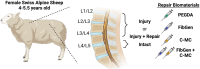




Similar articles
-
Composite biomaterial repair strategy to restore biomechanical function and reduce herniation risk in an ex vivo large animal model of intervertebral disc herniation with varying injury severity.PLoS One. 2019 May 28;14(5):e0217357. doi: 10.1371/journal.pone.0217357. eCollection 2019. PLoS One. 2019. PMID: 31136604 Free PMC article.
-
Ex-vivo biomechanics of repaired rat intervertebral discs using genipin crosslinked fibrin adhesive hydrogel.J Biomech. 2020 Dec 2;113:110100. doi: 10.1016/j.jbiomech.2020.110100. Epub 2020 Oct 28. J Biomech. 2020. PMID: 33142205 Free PMC article.
-
Genipin-crosslinked fibrin seeded with oxidized alginate microbeads as a novel composite biomaterial strategy for intervertebral disc cell therapy.Biomaterials. 2022 Aug;287:121641. doi: 10.1016/j.biomaterials.2022.121641. Epub 2022 Jun 17. Biomaterials. 2022. PMID: 35759923 Free PMC article.
-
Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair?Spine J. 2013 Mar;13(3):243-62. doi: 10.1016/j.spinee.2012.12.002. Epub 2013 Jan 29. Spine J. 2013. PMID: 23369494 Free PMC article. Review.
-
Biomaterials delivery strategies to repair degenerated intervertebral discs by regulating the inflammatory microenvironment.Front Immunol. 2023 Jan 23;14:1051606. doi: 10.3389/fimmu.2023.1051606. eCollection 2023. Front Immunol. 2023. PMID: 36756124 Free PMC article. Review.
Cited by
-
Advancing basic and preclinical spine research: Highlights from the ORS PSRS 6th International Spine Research Symposium.JOR Spine. 2023 Nov 21;6(4):e1308. doi: 10.1002/jsp2.1308. eCollection 2023 Dec. JOR Spine. 2023. PMID: 38156060 Free PMC article.
-
Regenerative potential of mouse neonatal intervertebral disc depends on collagen crosslink density.iScience. 2024 Sep 4;27(10):110883. doi: 10.1016/j.isci.2024.110883. eCollection 2024 Oct 18. iScience. 2024. PMID: 39319260 Free PMC article.
References
-
- Lo J, Chan L, Flynn S. A systematic review of the incidence, prevalence, costs, and activity and work limitations of amputation, osteoarthritis, rheumatoid arthritis, back pain, multiple sclerosis, spinal cord injury, stroke, and traumatic brain injury in the United States: a 2019 update. Arch Phys Med Rehabil. 2021;102:115‐131. - PMC - PubMed
-
- Vlaeyen JWS, Maher CG, Wiech K, et al. Low back pain. Nat Rev Dis Primers. 2018;4:52. - PubMed
-
- Yang H, Haldeman S. Behavior‐related factors associated with low Back pain in the US adult population. Spine. 2018;43:28‐34. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

