Prostate Safety Events During Testosterone Replacement Therapy in Men With Hypogonadism: A Randomized Clinical Trial
- PMID: 38150256
- PMCID: PMC10753401
- DOI: 10.1001/jamanetworkopen.2023.48692
Prostate Safety Events During Testosterone Replacement Therapy in Men With Hypogonadism: A Randomized Clinical Trial
Abstract
Importance: The effect of testosterone replacement therapy (TRT) on the risk of prostate cancer and other adverse prostate events is unknown.
Objective: To compare the effect of TRT vs placebo on the incidences of high-grade prostate cancers (Gleason score ≥4 + 3), any prostate cancer, acute urinary retention, invasive prostate procedures, and pharmacologic treatment for lower urinary tract symptoms in men with hypogonadism.
Design, setting, and participants: This placebo-controlled, double-blind randomized clinical trial enrolled 5246 men (aged 45-80 years) from 316 US trial sites who had 2 testosterone concentrations less than 300 ng/dL, hypogonadal symptoms, and cardiovascular disease (CVD) or increased CVD risk. Men with prostate-specific antigen (PSA) concentrations greater than 3.0 ng/mL and International Prostate Symptom Score (IPSS) greater than 19 were excluded. Enrollment took place between May 23, 2018, and February 1, 2022, and end-of-study visits were conducted between May 31, 2022, and January 19, 2023.
Intervention: Participants were randomized, with stratification for prior CVD, to topical 1.62% testosterone gel or placebo.
Main outcomes and measures: The primary prostate safety end point was the incidence of adjudicated high-grade prostate cancer. Secondary end points included incidence of any adjudicated prostate cancer, acute urinary retention, invasive prostate surgical procedure, prostate biopsy, and new pharmacologic treatment. Intervention effect was analyzed using a discrete-time proportional hazards model.
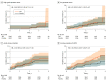
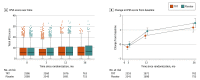
Results: A total of 5204 men (mean [SD] age, 63.3 [7.9] years) were analyzed. At baseline, the mean (SD) PSA concentration was 0.92 (0.67) ng/mL, and the mean (SD) IPSS was 7.1 (5.6). The mean (SD) treatment duration as 21.8 (14.2) months in the TRT group and 21.6 (14.0) months in the placebo group. During 14 304 person-years of follow-up, the incidence of high-grade prostate cancer (5 of 2596 [0.19%] in the TRT group vs 3 of 2602 [0.12%] in the placebo group; hazard ratio, 1.62; 95% CI, 0.39-6.77; P = .51) did not differ significantly between groups; the incidences of any prostate cancer, acute urinary retention, invasive surgical procedures, prostate biopsy, and new pharmacologic treatment also did not differ significantly. Change in IPSS did not differ between groups. The PSA concentrations increased more in testosterone-treated than placebo-treated men.
Conclusions and relevance: In a population of middle-aged and older men with hypogonadism, carefully evaluated to exclude those at high risk of prostate cancer, the incidences of high-grade or any prostate cancer and other prostate events were low and did not differ significantly between testosterone- and placebo-treated men. The study's findings may facilitate a more informed appraisal of the potential risks of TRT.
Trial registration: ClinicalTrials.gov Identifier: NCT03518034.
Conflict of interest statement
Figures

Similar articles
-
Efficacy of Testosterone Replacement Therapy in Correcting Anemia in Men With Hypogonadism: A Randomized Clinical Trial.JAMA Netw Open. 2023 Oct 2;6(10):e2340030. doi: 10.1001/jamanetworkopen.2023.40030. JAMA Netw Open. 2023. PMID: 37889486 Free PMC article. Clinical Trial.
-
Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial.JAMA. 2006 Nov 15;296(19):2351-61. doi: 10.1001/jama.296.19.2351. JAMA. 2006. PMID: 17105798 Clinical Trial.
-
Prostate Risk and Monitoring During Testosterone Replacement Therapy.J Clin Endocrinol Metab. 2024 Jul 12;109(8):1975-1983. doi: 10.1210/clinem/dgae334. J Clin Endocrinol Metab. 2024. PMID: 38753865 Review.
-
Testosterone treatment is not associated with increased risk of prostate cancer or worsening of lower urinary tract symptoms: prostate health outcomes in the Registry of Hypogonadism in Men.BJU Int. 2017 Feb;119(2):216-224. doi: 10.1111/bju.13578. Epub 2016 Aug 14. BJU Int. 2017. PMID: 27409523
-
Effects of Testosterone Replacement Therapy on Lower Urinary Tract Symptoms: A Systematic Review and Meta-analysis.Eur Urol. 2016 Jun;69(6):1083-90. doi: 10.1016/j.eururo.2016.01.043. Epub 2016 Feb 11. Eur Urol. 2016. PMID: 26874809 Review.
Cited by
-
Diagnosis and treatment of hypogonadism in men seeking to preserve fertility - what are the options?Int J Impot Res. 2024 Apr 30. doi: 10.1038/s41443-024-00897-4. Online ahead of print. Int J Impot Res. 2024. PMID: 38693209 Review.
-
Testosterone deficiency and chronic kidney disease.J Clin Transl Endocrinol. 2024 Aug 14;37:100365. doi: 10.1016/j.jcte.2024.100365. eCollection 2024 Sep. J Clin Transl Endocrinol. 2024. PMID: 39253627 Free PMC article. Review.
-
Testosterone therapy for functional hypogonadism in middle-aged and elderly males: current evidence and future perspectives.Hormones (Athens). 2024 Dec;23(4):801-817. doi: 10.1007/s42000-024-00587-2. Epub 2024 Jul 26. Hormones (Athens). 2024. PMID: 39060901 Review.
-
Age-related testosterone decline: mechanisms and intervention strategies.Reprod Biol Endocrinol. 2024 Nov 14;22(1):144. doi: 10.1186/s12958-024-01316-5. Reprod Biol Endocrinol. 2024. PMID: 39543598 Free PMC article. Review.
References
-
- Wang C, Nieschlag E, Swerdloff R, et al. ; International Society of Andrology (ISA); International Society for the Study of Aging Male (ISSAM); European Association of Urology (EAU); European Academy of Andrology (EAA); American Society of Andrology (ASA) . Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl. 2009;30(1):1-9. doi:10.2164/jandrol.108.006486 - DOI - PubMed
-
- Liverman C, Blazer DG, eds. Testosterone and Aging: Clinical Research Directions. National Academies Press; 2004. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

