Synergistic effect of hierarchical topographic structure on 3D-printed Titanium scaffold for enhanced coupling of osteogenesis and angiogenesis
- PMID: 38149019
- PMCID: PMC10750103
- DOI: 10.1016/j.mtbio.2023.100866
Synergistic effect of hierarchical topographic structure on 3D-printed Titanium scaffold for enhanced coupling of osteogenesis and angiogenesis
Abstract
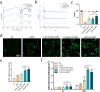
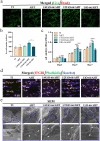
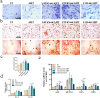
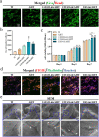
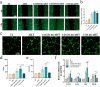
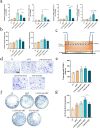
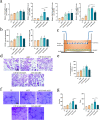
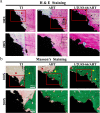
The significance of the osteogenesis-angiogenesis relationship in the healing process of bone defects has been increasingly emphasized in recent academic research. Surface topography plays a crucial role in guiding cellular behaviors. Metal-organic framework (MOF) is an innovative biomaterial with nanoscale structural and topological features, enabling the modulation of scaffold physicochemical properties. This study involved the loading of varying quantities of UiO-66 nanocrystals onto alkali-heat treated 3D-printed titanium scaffolds, resulting in the formation of hierarchical micro/nano topography named UiO-66/AHTs. The physicochemical properties of these scaffolds were subsequently characterized. Furthermore, the impact of these scaffolds on the osteogenic potential of BMSCs, the angiogenic potential of HUVECs, and their intercellular communication were investigated. The findings of this study indicated that 1/2UiO-66/AHT outperformed other groups in terms of osteogenic and angiogenic induction, as well as in promoting intercellular crosstalk by enhancing paracrine effects. These results suggest a promising biomimetic hierarchical topography design that facilitates the coupling of osteogenesis and angiogenesis.
Keywords: Coupling of osteogenesis and angiogenesis; Hierarchical topography; UiO-66.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study.Mater Sci Eng C Mater Biol Appl. 2021 Jan;118:111505. doi: 10.1016/j.msec.2020.111505. Epub 2020 Sep 11. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 33255064
-
3D printing of metal-organic framework incorporated porous scaffolds to promote osteogenic differentiation and bone regeneration.Nanoscale. 2020 Dec 23;12(48):24437-24449. doi: 10.1039/d0nr06297a. Nanoscale. 2020. PMID: 33305769
-
A low-temperature-printed hierarchical porous sponge-like scaffold that promotes cell-material interaction and modulates paracrine activity of MSCs for vascularized bone regeneration.Biomaterials. 2021 Jul;274:120841. doi: 10.1016/j.biomaterials.2021.120841. Epub 2021 Apr 30. Biomaterials. 2021. PMID: 33984633
-
Effect of nano-structured bioceramic surface on osteogenic differentiation of adipose derived stem cells.Biomaterials. 2014 Oct;35(30):8514-27. doi: 10.1016/j.biomaterials.2014.06.028. Epub 2014 Jul 4. Biomaterials. 2014. PMID: 25002263
-
Hierarchical bioceramic scaffolds with 3D-plotted macropores and mussel-inspired surface nanolayers for stimulating osteogenesis.Nanoscale. 2016 Jul 14;8(28):13790-803. doi: 10.1039/c6nr01952h. Nanoscale. 2016. PMID: 27380634
Cited by
-
Melatonin-encapsuled silk fibroin electrospun nanofibers promote vascularized bone regeneration through regulation of osteogenesis-angiogenesis coupling.Mater Today Bio. 2024 Feb 2;25:100985. doi: 10.1016/j.mtbio.2024.100985. eCollection 2024 Apr. Mater Today Bio. 2024. PMID: 38333049 Free PMC article.
References
LinkOut - more resources
Full Text Sources

