BETA prime: a first-in-man phase 1 study of AdAPT-001, an armed oncolytic adenovirus for solid tumors
- PMID: 38146006
- PMCID: PMC11016464
- DOI: 10.1038/s41417-023-00720-0
BETA prime: a first-in-man phase 1 study of AdAPT-001, an armed oncolytic adenovirus for solid tumors
Abstract
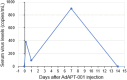
AdAPT-001 is an oncolytic adenovirus (OAV) with a transforming growth factor beta (TGF-ß) trap, which neutralizes the immunosuppressive and profibrotic cytokine, TGF-ß. The aim or purpose of this phase 1 study was to assess the safety and tolerability and, secondarily, the efficacy of AdAPT-001 after single intratumoral injection (IT) (Part 1) and multidose IT injection (Part 2) in patients with superficially accessible, advanced refractory solid tumors. Part 1 enrolled 9 patients with a 3 + 3 single dose-escalation safety run-in involving 2.5 × 1011, 5.0 × 1011, 1.0 × 1012 viral particles (vps). No dose-limiting toxicities or treatment-related serious adverse events (SAEs) were seen. In Part 2, a dose-expansion phase, 19 patients received AdAPT-001 at 1.0 × 1012 vps until disease progression according to Response Evaluation Criteria in Solid Tumors or RECIST 1.1. The overall responses to treatment included confirmed partial responses (3), durable stable disease ≥ 6 months (5), and progressive disease (13). AdAPT-001 is well tolerated. Evidence of an anti-tumor effect was seen in both injected and uninjected lesions. The recommended Phase 2 dose was 1.0 × 1012 vp administered by intratumoral injection once every 2 weeks. Combination of AdAPT-001 with a checkpoint inhibition is enrolling.
© 2023. The Author(s).
Conflict of interest statement
CL, TRR, SC, BO, MS, JW, EB, and AC report employment with and ownership of EpicentRx Inc. stock. No disclosures were reported by the other authors.
Figures
Similar articles
-
BETA PRIME: Phase I study of AdAPT-001 as monotherapy and combined with a checkpoint inhibitor in superficially accessible, treatment-refractory solid tumors.Future Oncol. 2022 Sep;18(29):3245-3254. doi: 10.2217/fon-2022-0481. Epub 2022 Aug 11. Future Oncol. 2022. PMID: 35950603
-
A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients.Gene Ther. 2009 Mar;16(3):376-82. doi: 10.1038/gt.2008.179. Epub 2008 Dec 25. Gene Ther. 2009. PMID: 19092859 Clinical Trial.
-
Preliminary efficacy and safety of YSCH-01 in patients with advanced solid tumors: an investigator-initiated trial.J Immunother Cancer. 2024 May 7;12(5):e008999. doi: 10.1136/jitc-2024-008999. J Immunother Cancer. 2024. PMID: 38719544 Free PMC article. Clinical Trial.
-
Evorpacept alone and in combination with pembrolizumab or trastuzumab in patients with advanced solid tumours (ASPEN-01): a first-in-human, open-label, multicentre, phase 1 dose-escalation and dose-expansion study.Lancet Oncol. 2021 Dec;22(12):1740-1751. doi: 10.1016/S1470-2045(21)00584-2. Epub 2021 Nov 15. Lancet Oncol. 2021. PMID: 34793719 Clinical Trial.
-
Intratumoral Immunotherapy-Update 2019.Oncologist. 2020 Mar;25(3):e423-e438. doi: 10.1634/theoncologist.2019-0438. Epub 2019 Nov 29. Oncologist. 2020. PMID: 32162802 Free PMC article. Review.
Cited by
-
Hypothesis: AdAPT-001 and pseudoprogression - when seeing is not necessarily believing.J Immunother Cancer. 2024 Jun 17;12(6):e008809. doi: 10.1136/jitc-2024-008809. J Immunother Cancer. 2024. PMID: 38886116 Free PMC article.
-
Translation of oncolytic viruses in sarcoma.Mol Ther Oncol. 2024 Jun 5;32(3):200822. doi: 10.1016/j.omton.2024.200822. eCollection 2024 Sep 19. Mol Ther Oncol. 2024. PMID: 39040851 Free PMC article. Review.
-
TGF-β trap of AdAPT-001 turns up the heat on tumors and turns down checkpoint blocker resistance.J Immunother Cancer. 2024 Oct 26;12(10):e009613. doi: 10.1136/jitc-2024-009613. J Immunother Cancer. 2024. PMID: 39461878 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

