Breaking the Silence: Regulation of HIV Transcription and Latency on the Road to a Cure
- PMID: 38140676
- PMCID: PMC10747579
- DOI: 10.3390/v15122435
Breaking the Silence: Regulation of HIV Transcription and Latency on the Road to a Cure
Abstract
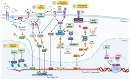
Antiretroviral therapy (ART) has brought the HIV/AIDS epidemic under control, but a curative strategy for viral eradication is still needed. The cessation of ART results in rapid viral rebound from latently infected CD4+ T cells, showing that control of viral replication alone does not fully restore immune function, nor does it eradicate viral reservoirs. With a better understanding of factors and mechanisms that promote viral latency, current approaches are primarily focused on the permanent silencing of latently infected cells ("block and lock") or reactivating HIV-1 gene expression in latently infected cells, in combination with immune restoration strategies to eliminate HIV infected cells from the host ("shock and kill"). In this review, we provide a summary of the current, most promising approaches for HIV-1 cure strategies, including an analysis of both latency-promoting agents (LPA) and latency-reversing agents (LRA) that have shown promise in vitro, ex vivo, and in human clinical trials to reduce the HIV-1 reservoir.
Keywords: HIV cure; LRA; block and lock; human immunodeficiency virus; latency reversal; shock and kill; viral latency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
NSC95397 Is a Novel HIV-1 Latency-Reversing Agent.Viruses. 2024 Nov 16;16(11):1783. doi: 10.3390/v16111783. Viruses. 2024. PMID: 39599897 Free PMC article.
-
Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.JBJS Essent Surg Tech. 2024 Dec 6;14(4):e23.00094. doi: 10.2106/JBJS.ST.23.00094. eCollection 2024 Oct-Dec. JBJS Essent Surg Tech. 2024. PMID: 39650795 Free PMC article.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
Epitranscriptomic m6A modifications during reactivation of HIV-1 latency in CD4+ T cells.mBio. 2024 Nov 13;15(11):e0221424. doi: 10.1128/mbio.02214-24. Epub 2024 Oct 7. mBio. 2024. PMID: 39373537 Free PMC article.
-
Sustained antiviral response against in vitro HIV-1 infection in peripheral blood mononuclear cells from people with chronic myeloid leukemia treated with ponatinib.Front Pharmacol. 2024 Sep 23;15:1426974. doi: 10.3389/fphar.2024.1426974. eCollection 2024. Front Pharmacol. 2024. PMID: 39380908 Free PMC article.
-
HIV Persistence, Latency, and Cure Approaches: Where Are We Now?Viruses. 2024 Jul 19;16(7):1163. doi: 10.3390/v16071163. Viruses. 2024. PMID: 39066325 Free PMC article. Review.
-
Integrator complex subunit 12 knockout overcomes a transcriptional block to HIV latency reversal.bioRxiv [Preprint]. 2025 Feb 19:2024.08.30.610517. doi: 10.1101/2024.08.30.610517. bioRxiv. 2025. PMID: 39257755 Free PMC article. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

