A Novel, Comprehensive A129 Mouse Model for Investigating Dengue Vaccines and Evaluating Pathogenesis
- PMID: 38140260
- PMCID: PMC10748371
- DOI: 10.3390/vaccines11121857
A Novel, Comprehensive A129 Mouse Model for Investigating Dengue Vaccines and Evaluating Pathogenesis
Abstract
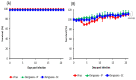
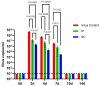
In search of a mouse model for use in evaluating dengue vaccines, we assessed A129 mice that lacked IFN-α/β receptors, rendering them susceptible to dengue virus (DENV) infection. To our knowledge, no reports have evaluated dengue vaccine efficiency using A129 mice. A129 mice were given a single intraperitoneal (IP) or subcutaneous (SC) injection of the vaccine, Dengvaxia. After 14 days of immunization via the IP or SC injection of Dengvaxia, the A129 mice exhibited notably elevated levels of anti-DENV immunoglobulin G and neutralizing antibodies (NAb) targeting all four DENV serotypes, with DENV-4 displaying the highest NAb levels. After challenge with DENV-2, Dengvaxia and mock-immunized mice survived, while only the mock group exhibited signs of morbidity. Viral genome levels in the serum and tissues (excluding the brain) were considerably lower in the immunized mice compared to those in the mock group. The SC administration of Dengvaxia resulted in lower viremia levels than IP administration did. Therefore, given that A129 mice manifest dengue-related morbidity, including viremia in the serum and other tissues, these mice represent a valuable model for investigating novel dengue vaccines and antiviral drugs and for exploring dengue pathogenesis.
Keywords: A129 mouse; Dengvaxia vaccine; dengue virus; neutralization antibody; viral loads.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Noninvasive bioluminescence imaging of dengue virus infection in the brain of A129 mice.Appl Microbiol Biotechnol. 2013 May;97(10):4589-96. doi: 10.1007/s00253-013-4799-8. Epub 2013 Mar 7. Appl Microbiol Biotechnol. 2013. PMID: 23467830
-
Dengue and Zika virus infections are enhanced by live attenuated dengue vaccine but not by recombinant DSV4 vaccine candidate in mouse models.EBioMedicine. 2020 Oct;60:102991. doi: 10.1016/j.ebiom.2020.102991. Epub 2020 Sep 16. EBioMedicine. 2020. PMID: 32949997 Free PMC article.
-
Immunodomination of Serotype-Specific CD4+ T-Cell Epitopes Contributed to the Biased Immune Responses Induced by a Tetravalent Measles-Vectored Dengue Vaccine.Front Immunol. 2020 Mar 31;11:546. doi: 10.3389/fimmu.2020.00546. eCollection 2020. Front Immunol. 2020. PMID: 32300346 Free PMC article.
-
Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine.Front Cell Infect Microbiol. 2020 Oct 22;10:572681. doi: 10.3389/fcimb.2020.572681. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33194810 Free PMC article. Review.
-
Does structurally-mature dengue virion matter in vaccine preparation in post-Dengvaxia era?Hum Vaccin Immunother. 2019;15(10):2328-2336. doi: 10.1080/21645515.2019.1643676. Epub 2019 Aug 23. Hum Vaccin Immunother. 2019. PMID: 31314657 Free PMC article. Review.
Cited by
-
The Dynamic Relationship between Dengue Virus and the Human Cutaneous Innate Immune Response.Viruses. 2024 May 4;16(5):727. doi: 10.3390/v16050727. Viruses. 2024. PMID: 38793609 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

