Combating Black Fungus: Using Allicin as a Potent Antifungal Agent against Mucorales
- PMID: 38139348
- PMCID: PMC10743604
- DOI: 10.3390/ijms242417519
Combating Black Fungus: Using Allicin as a Potent Antifungal Agent against Mucorales
Abstract
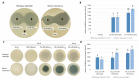
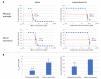
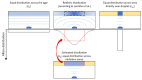
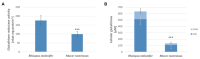
Invasive fungal (IF) diseases are a leading global cause of mortality, particularly among immunocompromised individuals. The SARS-CoV-2 pandemic further exacerbated this scenario, intensifying comorbid IF infections such as mucormycoses of the nasopharynx. In the work reported here, it is shown that zygomycetes, significant contributors to mycoses, are sensitive to the natural product allicin. Inhibition of Mucorales fungi by allicin in solution and by allicin vapor was demonstrated. Mathematical modeling showed that the efficacy of allicin vapor is comparable to direct contact with the commercially available antifungal agent amphotericin B (ampB). Furthermore, the study revealed a synergistic interaction between allicin and the non-volatile ampB. The toxicity of allicin solution to human cell lines was evaluated and it was found that the half maximal effective concentration (EC50) of allicin was 25-72 times higher in the cell lines as compared to the fungal spores. Fungal allicin sensitivity depends on the spore concentration, as demonstrated in a drop test. This study shows the potential of allicin, a sulfur-containing defense compound from garlic, to combat zygomycete fungi. The findings underscore allicin's promise for applications in infections of the nasopharynx via inhalation, suggesting a novel therapeutic avenue against challenging fungal infections.
Keywords: COVID-19; allicin; amphotericin B; antimycotic; glutathione reductase; mucormycosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Allicin as a Volatile or Nebulisable Antimycotic for the Treatment of Pulmonary Mycoses: In Vitro Studies Using a Lung Flow Test Rig.Int J Mol Sci. 2022 Jun 14;23(12):6607. doi: 10.3390/ijms23126607. Int J Mol Sci. 2022. PMID: 35743050 Free PMC article.
-
An overview of the antifungal properties of allicin and its breakdown products--the possibility of a safe and effective antifungal prophylactic.Mycoses. 2005 Mar;48(2):95-100. doi: 10.1111/j.1439-0507.2004.01076.x. Mycoses. 2005. PMID: 15743425 Review.
-
Allicin enhances the oxidative damage effect of amphotericin B against Candida albicans.Int J Antimicrob Agents. 2009 Mar;33(3):258-63. doi: 10.1016/j.ijantimicag.2008.09.014. Epub 2008 Dec 17. Int J Antimicrob Agents. 2009. PMID: 19095412
-
The vacuole-targeting fungicidal activity of amphotericin B against the pathogenic fungus Candida albicans and its enhancement by allicin.J Antibiot (Tokyo). 2009 Dec;62(12):691-7. doi: 10.1038/ja.2009.103. Epub 2009 Oct 30. J Antibiot (Tokyo). 2009. PMID: 19876074
-
Revitalizing allicin for cancer therapy: advances in formulation strategies to enhance bioavailability, stability, and clinical efficacy.Naunyn Schmiedebergs Arch Pharmacol. 2024 Feb;397(2):703-724. doi: 10.1007/s00210-023-02675-3. Epub 2023 Aug 24. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37615709 Review.
References
-
- World Health Organization . WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Volume 1. WHO; Geneva, Switzerland: 2022.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

