Granulocyte-Macrophage-Colony-Stimulating-Factor Combined with Prostaglandin E1 Create Dendritic Cells of Leukemic Origin from AML Patients' Whole Blood and Whole Bone Marrow That Mediate Antileukemic Processes after Mixed Lymphocyte Culture
- PMID: 38139264
- PMCID: PMC10743754
- DOI: 10.3390/ijms242417436
Granulocyte-Macrophage-Colony-Stimulating-Factor Combined with Prostaglandin E1 Create Dendritic Cells of Leukemic Origin from AML Patients' Whole Blood and Whole Bone Marrow That Mediate Antileukemic Processes after Mixed Lymphocyte Culture
Abstract
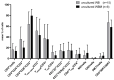
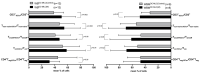
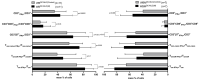
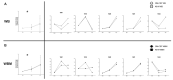
Although several (chemotherapeutic) protocols to treat acute myeloid leukemia (AML) are available, high rates of relapses in successfully treated patients occur. Strategies to stabilize remissions are greatly needed. The combination of the (clinically approved) immune-modulatory compounds Granulocyte-Macrophage-Colony-Stimulating-Factor (GM-CSF) and Prostaglandine E1 (PGE-1) (Kit-M) converts myeloid blasts into dendritic cells of leukemic origin (DCleu). After stimulation with DCleu ex vivo, leukemia-specific antileukemic immune cells are activated. Therefore, Kit-M treatment may be an attractive immunotherapeutic tool to treat patients with myeloid leukemia. Kit-M-mediated antileukemic effects on whole bone marrow (WBM) were evaluated and compared to whole blood (WB) to evaluate the potential effects of Kit-M on both compartments. WB and WBM samples from 17 AML patients at first diagnosis, in persisting disease and at relapse after allogeneic stem cell transplantation (SCT) were treated in parallel with Kit-M to generate DC/DCleu. Untreated samples served as controls. After a mixed lymphocyte culture enriched with patients' T cells (MLC), the leukemia-specific antileukemic effects were assessed through the degranulation- (CD107a+ T cells), the intracellular IFNγ production- and the cytotoxicity fluorolysis assay. Quantification of cell subtypes was performed via flow cytometry. In both WB and WBM significantly higher frequencies of (mature) DCleu were generated without induction of blast proliferation in Kit-M-treated samples compared to control. After MLC with Kit-M-treated vs. not pretreated WB or WBM, frequencies of (leukemia-specific) immunoreactive cells (e.g., non-naive, effector-, memory-, CD3+β7+ T cells, NK- cells) were (significantly) increased, whereas leukemia-specific regulatory T cells (Treg, CD152+ T cells) were (significantly) decreased. The cytotoxicity fluorolysis assay showed a significantly improved blast lysis in Kit-M-treated WB and WBM compared to control. A parallel comparison of WB and WBM samples revealed no significant differences in frequencies of DCleu, (leukemia-specific) immunoreactive cells and achieved antileukemic processes. Kit-M was shown to have comparable effects on WB and WBM samples regarding the generation of DCleu and activation of (antileukemic) immune cells after MLC. This was true for samples before or after SCT. In summary, a potential Kit-M in vivo treatment could lead to antileukemic effects in WB as well as WBM in vivo and to stabilization of the disease or remission in patients before or after SCT. A clinical trial is currently being planned.
Keywords: AML; dendritic cell-based therapy; immune escape; leukemia-derived dendritic cells; whole blood; whole bone marrow.
Conflict of interest statement
H.M.S. is involved with Modiblast Pharma GmbH (Oberhaching, Germany), which holds the European Patent 15 801 987.7-1118 and US Patent 15-517627, “Use of immunomod-ulatory effective compositions for the immunotherapeutic treatment of patients suffering from myeloid leukemias”.
Figures






Similar articles
-
Effective and Successful Quantification of Leukemia-Specific Immune Cells in AML Patients' Blood or Culture, Focusing on Intracellular Cytokine and Degranulation Assays.Int J Mol Sci. 2024 Jun 26;25(13):6983. doi: 10.3390/ijms25136983. Int J Mol Sci. 2024. PMID: 39000091 Free PMC article.
-
Dendritic Cell-Triggered Immune Activation Goes along with Provision of (Leukemia-Specific) Integrin Beta 7-Expressing Immune Cells and Improved Antileukemic Processes.Int J Mol Sci. 2022 Dec 27;24(1):463. doi: 10.3390/ijms24010463. Int J Mol Sci. 2022. PMID: 36613907 Free PMC article.
-
Leukemia derived dendritic cell (DCleu) mediated immune response goes along with reduced (leukemia-specific) regulatory T-cells.Immunobiology. 2022 Jul;227(4):152237. doi: 10.1016/j.imbio.2022.152237. Epub 2022 Jun 11. Immunobiology. 2022. PMID: 35749805
-
Dendritic Cells of Leukemic Origin: Specialized Antigen-Presenting Cells as Potential Treatment Tools for Patients with Myeloid Leukemia.Transfus Med Hemother. 2020 Dec;47(6):432-443. doi: 10.1159/000512452. Epub 2020 Nov 5. Transfus Med Hemother. 2020. PMID: 33442338 Free PMC article. Review.
-
Expression of FLT3 receptor and response to FLT3 ligand by leukemic cells.Leukemia. 1996 Apr;10(4):588-99. Leukemia. 1996. PMID: 8618433 Review.
Cited by
-
Anti-Leukemic Effects Induced by Dendritic Cells of Leukemic Origin from Leukemic Blood Samples Are Comparable under Hypoxic vs. Normoxic Conditions.Cancers (Basel). 2024 Jun 28;16(13):2383. doi: 10.3390/cancers16132383. Cancers (Basel). 2024. PMID: 39001445 Free PMC article.
-
Effective and Successful Quantification of Leukemia-Specific Immune Cells in AML Patients' Blood or Culture, Focusing on Intracellular Cytokine and Degranulation Assays.Int J Mol Sci. 2024 Jun 26;25(13):6983. doi: 10.3390/ijms25136983. Int J Mol Sci. 2024. PMID: 39000091 Free PMC article.
References
-
- Pelcovits A., Niroula R. Acute Myeloid Leukemia: A Review. Rhode Isl. Med. J. 2020;103:38–40. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

