Positive Regulation of S-Adenosylmethionine on Chondrocytic Differentiation via Stimulation of Polyamine Production and the Gene Expression of Chondrogenic Differentiation Factors
- PMID: 38139122
- PMCID: PMC10743985
- DOI: 10.3390/ijms242417294
Positive Regulation of S-Adenosylmethionine on Chondrocytic Differentiation via Stimulation of Polyamine Production and the Gene Expression of Chondrogenic Differentiation Factors
Abstract
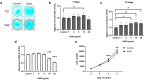
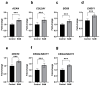
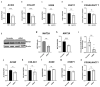
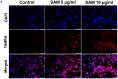
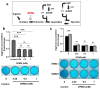
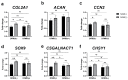
S-adenosylmethionine (SAM) is considered to be a useful therapeutic agent for degenerative cartilage diseases, although its mechanism is not clear. We previously found that polyamines stimulate the expression of differentiated phenotype of chondrocytes. We also found that the cellular communication network factor 2 (CCN2) played a huge role in the proliferation and differentiation of chondrocytes. Therefore, we hypothesized that polyamines and CCN2 could be involved in the chondroprotective action of SAM. In this study, we initially found that exogenous SAM enhanced proteoglycan production but not cell proliferation in human chondrocyte-like cell line-2/8 (HCS-2/8) cells. Moreover, SAM enhanced gene expression of cartilage-specific matrix (aggrecan and type II collagen), Sry-Box transcription factor 9 (SOX9), CCN2, and chondroitin sulfate biosynthetic enzymes. The blockade of the methionine adenosyltransferase 2A (MAT2A) enzyme catalyzing intracellular SAM biosynthesis restrained the effect of SAM on chondrocytes. The polyamine level in chondrocytes was higher in SAM-treated culture than control culture. Additionally, Alcian blue staining and RT-qPCR indicated that the effects of SAM on the production and gene expression of aggrecan were reduced by the inhibition of polyamine synthesis. These results suggest that the stimulation of polyamine synthesis and gene expression of chondrogenic differentiation factors, such as CCN2, account for the mechanism underlying the action of SAM on chondrocytes.
Keywords: CCN2; ODC; S-adenosylmethionine; chondrocyte differentiation; gene expression; polyamine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Novel chondrogenic and chondroprotective effects of the natural compound harmine.Biochimie. 2013 Feb;95(2):374-81. doi: 10.1016/j.biochi.2012.10.016. Epub 2012 Oct 29. Biochimie. 2013. PMID: 23116713
-
Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis.J Hepatol. 2013 Oct;59(4):830-41. doi: 10.1016/j.jhep.2013.04.031. Epub 2013 May 7. J Hepatol. 2013. PMID: 23665184 Review.
-
Abscisic acid, H2O2 and nitric oxide interactions mediated cold-induced S-adenosylmethionine synthetase in Medicago sativa subsp. falcata that confers cold tolerance through up-regulating polyamine oxidation.Plant Biotechnol J. 2014 Jun;12(5):601-12. doi: 10.1111/pbi.12166. Epub 2014 Feb 12. Plant Biotechnol J. 2014. PMID: 24517136
-
Flavonoid Compound Icariin Activates Hypoxia Inducible Factor-1α in Chondrocytes and Promotes Articular Cartilage Repair.PLoS One. 2016 Feb 3;11(2):e0148372. doi: 10.1371/journal.pone.0148372. eCollection 2016. PLoS One. 2016. PMID: 26841115 Free PMC article.
-
Methionine salvage and S-adenosylmethionine: essential links between sulfur, ethylene and polyamine biosynthesis.Biochem J. 2013 Apr 15;451(2):145-54. doi: 10.1042/BJ20121744. Biochem J. 2013. PMID: 23535167 Review.
References
-
- Toennies G. Sulfonium reactions of methionine and their possible metabolic significance. J. Biol. Chem. 1940;132:455–456. doi: 10.1016/S0021-9258(18)73428-5. - DOI
-
- Zhang Y., Ma R., Deng Q., Wang W., Cao C., Yu C., Li S., Shi L., Tian J. S-Adenosylmethionine Improves Cognitive Impairment in D-Galactose-Induced Brain Aging by Inhibiting Oxidative Stress and Neuroinflammation. J. Chem. Neuroanat. 2023;128:102232. doi: 10.1016/j.jchemneu.2023.102232. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

