Anionic Hyperbranched Amphiphilic Polyelectrolytes as Nanocarriers for Antimicrobial Proteins and Peptides
- PMID: 38138846
- PMCID: PMC10745097
- DOI: 10.3390/ma16247702
Anionic Hyperbranched Amphiphilic Polyelectrolytes as Nanocarriers for Antimicrobial Proteins and Peptides
Abstract
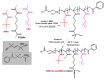
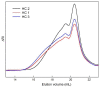
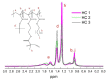
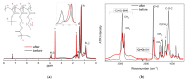
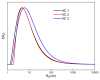
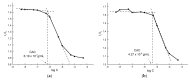
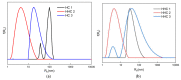
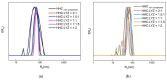
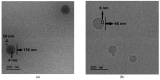
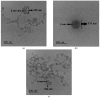
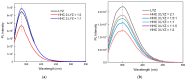
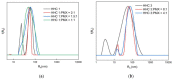
This manuscript presents the synthesis of hyperbranched amphiphilic poly (lauryl methacrylate-co-tert-butyl methacrylate-co-methacrylic acid), H-P(LMA-co-tBMA-co-MAA) copolymers via reversible addition fragmentation chain transfer (RAFT) copolymerization of tBMA and LMA, and their post-polymerization modification to anionic amphiphilic polyelectrolytes. The focus is on investigating whether the combination of the hydrophobic characters of LMA and tBMA segments, as well as the polyelectrolyte and hydrophilic properties of MAA segments, both distributed within a unique hyperbranched polymer chain topology, would result in intriguing, branched copolymers with the potential to be applied in nanomedicine. Therefore, we studied the self-assembly behavior of these copolymers in aqueous media, as well as their ability to form complexes with cationic proteins, namely lysozyme (LYZ) and polymyxin (PMX). Various physicochemical characterization techniques, including size exclusion chromatography (SEC) and proton nuclear magnetic resonance (1H-NMR), verified the molecular characteristics of these well-defined copolymers, whereas light scattering and fluorescence spectroscopy techniques revealed promising nanoparticle (NP) self- and co-assembly properties of the copolymers in aqueous media.
Keywords: amphiphilic copolymers; hyperbranched; nanoparticles; polyelectrolytes; protein complexation; self-assembly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Hyperbranched Polyelectrolyte Copolymers as Novel Candidate Delivery Systems for Bio-Relevant Compounds.Materials (Basel). 2023 Jan 24;16(3):1045. doi: 10.3390/ma16031045. Materials (Basel). 2023. PMID: 36770053 Free PMC article.
-
Hyperbranched Copolymers of Methacrylic Acid and Lauryl Methacrylate H-P(MAA-co-LMA): Synthetic Aspects and Interactions with Biorelevant Compounds.Pharmaceutics. 2023 Apr 9;15(4):1198. doi: 10.3390/pharmaceutics15041198. Pharmaceutics. 2023. PMID: 37111683 Free PMC article.
-
Four-Component Statistical Copolymers by RAFT Polymerization.Polymers (Basel). 2024 May 8;16(10):1321. doi: 10.3390/polym16101321. Polymers (Basel). 2024. PMID: 38794514 Free PMC article.
-
The Role of Polymer Structure in Formation of Various Nano- and Microstructural Materials: 30 Years of Research in the Laboratory of Nano- and Microstructural Materials at the Centre of Polymer and Carbon Materials PAS.Polymers (Basel). 2021 Aug 27;13(17):2892. doi: 10.3390/polym13172892. Polymers (Basel). 2021. PMID: 34502932 Free PMC article. Review.
-
Polymeric Nanostructures Containing Proteins and Peptides for Pharmaceutical Applications.Polymers (Basel). 2022 Feb 16;14(4):777. doi: 10.3390/polym14040777. Polymers (Basel). 2022. PMID: 35215689 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

