Clinical Evaluation of Direct Reverse Transcription PCR for Detection of SARS-CoV-2 Compared to Conventional RT-PCR in Patients with Positive Rapid Antigen Test Results during Circulation of Emerging Viral Variants
- PMID: 38132252
- PMCID: PMC10743042
- DOI: 10.3390/diagnostics13243668
Clinical Evaluation of Direct Reverse Transcription PCR for Detection of SARS-CoV-2 Compared to Conventional RT-PCR in Patients with Positive Rapid Antigen Test Results during Circulation of Emerging Viral Variants
Abstract
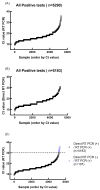
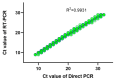
The emergence of the Omicron (B.1.1.529) variant of SARS-CoV-2 has precipitated a new global wave of the COVID-19 pandemic. The rapid identification of SARS-CoV-2 infection is imperative for the effective mitigation of transmission. Diagnostic modalities such as rapid antigen testing and real-time reverse transcription polymerase chain reaction (RT-PCR) offer expedient turnaround times of 10-15 min and straightforward implementation. This preliminary study assessed the correlation between outcomes of commercially available rapid antigen tests for home use and conventional reverse transcription polymerase chain reaction (RT-PCR) assays using a limited set of clinical specimens. Patients aged 5-99 years presenting to the emergency department for SARS-CoV-2 testing were eligible for enrollment (n = 5652). Direct PCR and conventional RT-PCR were utilized for the detection of SARS-CoV-2. The entire cohort of 5652 clinical specimens was assessed by both modalities to determine the clinical utility of the direct RT-PCR assay. Timely confirmation of SARS-CoV-2 infection may attenuate viral propagation and guide therapeutic interventions. Additionally, direct RT-PCR as a secondary confirmatory test for at-home rapid antigen test results demonstrated sensitivity comparable to conventional RT-PCR, indicating utility for implementation in laboratories globally, especially in resource-limited settings with constraints on reagents, equipment, and skilled personnel. In summary, direct RT-PCR enables the detection of SARS-CoV-2 with a sensitivity approaching that of conventional RT-PCR while offering expedient throughput and shorter turnaround times. Moreover, direct RT-PCR provides an open-source option for diagnostic laboratories worldwide, particularly in low- and middle-income countries.
Keywords: COVID-19; antigen test; at-home kit; direct RT-PCR; omicron variant.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the study design, data collection, interpretation, or decision to submit the work for publication.
Figures
Similar articles
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article. Review.
-
[Evaluation of the Rapid Antigen Detection Kit with the Polymerase Chain Reaction for Detection of SARS-CoV-2 in Respiratory Samples].Mikrobiyol Bul. 2022 Apr;56(2):263-273. doi: 10.5578/mb.20229806. Mikrobiyol Bul. 2022. PMID: 35477229 Turkish.
-
Clinical assessment of SARS-CoV-2 antigen rapid detection compared with RT-PCR assay for emerging variants at a high-throughput community testing site in Taiwan.Int J Infect Dis. 2022 Feb;115:30-34. doi: 10.1016/j.ijid.2021.11.034. Epub 2021 Nov 26. Int J Infect Dis. 2022. PMID: 34843956 Free PMC article.
-
Comparison of Rapid Antigen Tests' Performance Between Delta and Omicron Variants of SARS-CoV-2 : A Secondary Analysis From a Serial Home Self-testing Study.Ann Intern Med. 2022 Dec;175(12):1685-1692. doi: 10.7326/M22-0760. Epub 2022 Oct 11. Ann Intern Med. 2022. PMID: 36215709 Free PMC article.
-
Conventional and Novel Diagnostic Tools for the Diagnosis of Emerging SARS-CoV-2 Variants.Vaccines (Basel). 2023 Feb 6;11(2):374. doi: 10.3390/vaccines11020374. Vaccines (Basel). 2023. PMID: 36851252 Free PMC article. Review.
References
-
- van Ogtrop M.L., van de Laar T.J.W., Eggink D., Vanhommerig J.W., van der Reijden W.A. Comparison of the performance of the PanBio COVID-19 antigen test in SARS-CoV-2 B.1.1.7 (alpha) variants versus non-B.1.1.7 variants. Microbiol. Spectr. 2021;9:e0088421. doi: 10.1128/Spectrum.00884-21. - DOI - PMC - PubMed
-
- Alkhatib M., Salpini R., Carioti L., Ambrosio F.A., D’Anna S., Duca L., Costa G., Bellocchi M.C., Piermatteo L., Artese A., et al. Update on SARS-CoV-2 Omicron variant of concern and its peculiar mutational profile. Microbiol. Spectr. 2022;10:e0273221. doi: 10.1128/spectrum.02732-21. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

