ENPP1 is an innate immune checkpoint of the anticancer cGAMP-STING pathway in breast cancer
- PMID: 38117852
- PMCID: PMC10756298
- DOI: 10.1073/pnas.2313693120
ENPP1 is an innate immune checkpoint of the anticancer cGAMP-STING pathway in breast cancer
Abstract
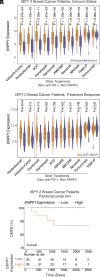
Ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) expression correlates with poor prognosis in many cancers, and we previously discovered that ENPP1 is the dominant hydrolase of extracellular cGAMP: a cancer-cell-produced immunotransmitter that activates the anticancer stimulator of interferon genes (STING) pathway. However, ENPP1 has other catalytic activities and the molecular and cellular mechanisms contributing to its tumorigenic effects remain unclear. Here, using single-cell RNA-seq, we show that ENPP1 in both cancer and normal tissues drives primary breast tumor growth and metastasis by dampening extracellular 2'3'-cyclic-GMP-AMP (cGAMP)-STING-mediated antitumoral immunity. ENPP1 loss-of-function in both cancer cells and normal tissues slowed primary tumor growth and abolished metastasis. Selectively abolishing the cGAMP hydrolysis activity of ENPP1 phenocopied ENPP1 knockout in a STING-dependent manner, demonstrating that restoration of paracrine cGAMP-STING signaling is the dominant anti-cancer mechanism of ENPP1 inhibition. Finally, ENPP1 expression in breast tumors deterministically predicated whether patients would remain free of distant metastasis after pembrolizumab (anti-PD-1) treatment followed by surgery. Altogether, ENPP1 blockade represents a strategy to exploit cancer-produced extracellular cGAMP for controlled local activation of STING and is therefore a promising therapeutic approach against breast cancer.
Keywords: ENPP1; STING; breast cancer metastasis; extracellular cGAMP; immune checkpoint.
Conflict of interest statement
Competing interests statement:V.B. and L.L. have filed two patents on ENPP1 inhibitors (PCT/US2020/015968 and PCT/US2018/050018) that are licensed to Angarus Therapeutics. L.L. is a co-founder of Angarus Therapeutics. L.A.G. has filed patents on CRISPR technologies and is a co-founder of Chroma Medicine. All other authors have no additional financial interests.
Figures


Update of
-
ENPP1 is an innate immune checkpoint of the anticancer cGAMP-STING pathway.bioRxiv [Preprint]. 2023 Jun 5:2023.06.01.543353. doi: 10.1101/2023.06.01.543353. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2023 Dec 26;120(52):e2313693120. doi: 10.1073/pnas.2313693120 PMID: 37333273 Free PMC article. Updated. Preprint.
Similar articles
-
ENPP1 is an innate immune checkpoint of the anticancer cGAMP-STING pathway.bioRxiv [Preprint]. 2023 Jun 5:2023.06.01.543353. doi: 10.1101/2023.06.01.543353. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2023 Dec 26;120(52):e2313693120. doi: 10.1073/pnas.2313693120 PMID: 37333273 Free PMC article. Updated. Preprint.
-
Identification of the extracellular membrane protein ENPP3 as a major cGAMP hydrolase and innate immune checkpoint.Cell Rep. 2024 May 28;43(5):114209. doi: 10.1016/j.celrep.2024.114209. Epub 2024 May 14. Cell Rep. 2024. PMID: 38749434
-
Tumor Exosomal ENPP1 Hydrolyzes cGAMP to Inhibit cGAS-STING Signaling.Adv Sci (Weinh). 2024 May;11(20):e2308131. doi: 10.1002/advs.202308131. Epub 2024 Mar 18. Adv Sci (Weinh). 2024. PMID: 38498770 Free PMC article.
-
ENPP1, an Old Enzyme with New Functions, and Small Molecule Inhibitors-A STING in the Tale of ENPP1.Molecules. 2019 Nov 19;24(22):4192. doi: 10.3390/molecules24224192. Molecules. 2019. PMID: 31752288 Free PMC article. Review.
-
Ecto-nucleotide pyrophosphatase/phosphodiesterase 1 inhibitors: Research progress and prospects.Eur J Med Chem. 2024 Mar 5;267:116211. doi: 10.1016/j.ejmech.2024.116211. Epub 2024 Feb 10. Eur J Med Chem. 2024. PMID: 38359537 Review.
Cited by
-
Small molecule innate immune modulators in cancer therapy.Front Immunol. 2024 Sep 10;15:1395655. doi: 10.3389/fimmu.2024.1395655. eCollection 2024. Front Immunol. 2024. PMID: 39318624 Free PMC article. Review.
-
Cold and hot tumors: from molecular mechanisms to targeted therapy.Signal Transduct Target Ther. 2024 Oct 18;9(1):274. doi: 10.1038/s41392-024-01979-x. Signal Transduct Target Ther. 2024. PMID: 39420203 Free PMC article. Review.
-
Investigating the impact of STING pathway activation on breast cancer treatment outcomes: development and validation of a prognostic model.Front Immunol. 2024 Aug 9;15:1438364. doi: 10.3389/fimmu.2024.1438364. eCollection 2024. Front Immunol. 2024. PMID: 39185402 Free PMC article.
-
Second messenger 2'3'-cyclic GMP-AMP (2'3'-cGAMP): the cell autonomous and non-autonomous roles in cancer progression.Acta Pharmacol Sin. 2024 May;45(5):890-899. doi: 10.1038/s41401-023-01210-7. Epub 2024 Jan 4. Acta Pharmacol Sin. 2024. PMID: 38177693 Review.
-
Associations of Immune Checkpoint Predictive Biomarkers (MHC-I and MHC-II) with Clinical and Molecular Features in a Diverse Breast Cancer Cohort.Clin Cancer Res. 2024 Sep 13;30(18):4077-4081. doi: 10.1158/1078-0432.CCR-24-1286. Clin Cancer Res. 2024. PMID: 39007881
References
-
- Garon E. B., et al. , Pembrolizumab for the treatment of non–small-cell lung cancer’. N. Engl. J. Med. 372, 2018–2028 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

