Conformationally Restricted Analogues of α-Galactosylceramide as Adjuvant in COVID-19 Subunit Vaccine
- PMID: 38116441
- PMCID: PMC10726466
- DOI: 10.1021/acsmedchemlett.3c00154
Conformationally Restricted Analogues of α-Galactosylceramide as Adjuvant in COVID-19 Subunit Vaccine
Abstract
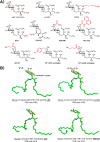
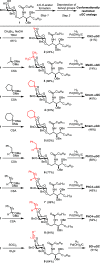
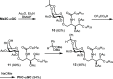
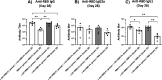
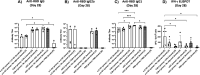
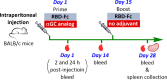
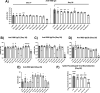
iNKT cells are a type of T lymphocyte that recognizes glycolipid antigens presented by CD1d protein. αGC is an agonistic glycolipid that activates iNKT cells and triggers immune modulatory cytokine responses, making it a promising vaccine adjuvant. To find more potent immunostimulating glycolipids, we prepared 4,6-O-galactosyl conformationally restricted analogues of αGC. Mice vaccinated with the SARS-CoV-2 RBD-Fc vaccine adjuvanted with these newly developed glycolipids produced robust anti-RBD antibody responses, comparable to those achieved with αGC. Importantly, we also found that omitting αGC, α-C-GalCer (Th1-type agonist), or C20:2 (Th2-type agonist) from the booster vaccine had negligible impact on antibody and cellular responses, potentially reducing the frequency of adjuvant use required to maintain potent immune responses.
© 2023 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
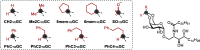
Similar articles
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

