Dysregulated Autophagy and Sarcomere Dysfunction in Patients With Heart Failure With Co-Occurrence of P63A and P380S BAG3 Variants
- PMID: 38108245
- PMCID: PMC10863766
- DOI: 10.1161/JAHA.123.029938
Dysregulated Autophagy and Sarcomere Dysfunction in Patients With Heart Failure With Co-Occurrence of P63A and P380S BAG3 Variants
Abstract
Background: Mutations to the co-chaperone protein BAG3 (B-cell lymphoma-2-associated athanogene-3) are a leading cause of dilated cardiomyopathy (DCM). These mutations often impact the C-terminal BAG domain (residues 420-499), which regulates heat shock protein 70-dependent protein turnover via autophagy. While mutations in other regions are less common, previous studies in patients with DCM found that co-occurrence of 2 BAG3 variants (P63A, P380S) led to worse prognosis. However, the underlying mechanism for dysfunction is not fully understood.
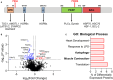
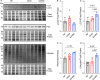
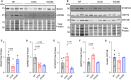
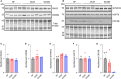
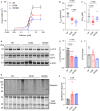
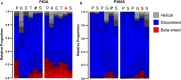
Methods and results: In this study, we used proteomics, Western blots, and myofilament functional assays on left ventricular tissue from patients with nonfailing, DCM, and DCM with BAG363/380 to determine how these mutations impact protein quality control and cardiomyocyte contractile function. We found dysregulated autophagy and increased protein ubiquitination in patients with BAG363/380 compared with nonfailing and DCM, suggesting impaired protein turnover. Expression and myofilament localization of BAG3-binding proteins were also uniquely altered in the BAG3,63/380 including abolished localization of the small heat shock protein CRYAB (alpha-crystallin B chain) to the sarcomere. To determine whether these variants impacted sarcomere function, we used cardiomyocyte force-calcium assays and found reduced maximal calcium-activated force in DCM and BAG363/380. Interestingly, myofilament calcium sensitivity was increased in DCM but not with BAG363/380, which was not explained by differences in troponin I phosphorylation.
Conclusions: Together, our data support that the disease-enhancing mechanism for BAG3 variants outside of the BAG domain is through disrupted protein turnover leading to compromised sarcomere function. These findings suggest a shared mechanism of disease among pathogenic BAG3 variants, regardless of location.
Keywords: BAG3; autophagy; dilated cardiomyopathy; sarcomere.
Figures
Similar articles
-
BAG3 expression and sarcomere localization in the human heart are linked to HSF-1 and are differentially affected by sex and disease.Am J Physiol Heart Circ Physiol. 2021 Jun 1;320(6):H2339-H2350. doi: 10.1152/ajpheart.00419.2020. Epub 2021 May 14. Am J Physiol Heart Circ Physiol. 2021. PMID: 33989081 Free PMC article.
-
Cardiomyocyte contractile impairment in heart failure results from reduced BAG3-mediated sarcomeric protein turnover.Nat Commun. 2021 May 19;12(1):2942. doi: 10.1038/s41467-021-23272-z. Nat Commun. 2021. PMID: 34011988 Free PMC article.
-
Dilated Cardiomyopathy Due to BLC2-Associated Athanogene 3 (BAG3) Mutations.J Am Coll Cardiol. 2018 Nov 13;72(20):2471-2481. doi: 10.1016/j.jacc.2018.08.2181. J Am Coll Cardiol. 2018. PMID: 30442290 Free PMC article.
-
Advances in the role and mechanism of BAG3 in dilated cardiomyopathy.Heart Fail Rev. 2021 Jan;26(1):183-194. doi: 10.1007/s10741-019-09899-7. Heart Fail Rev. 2021. PMID: 31808029 Review.
-
The role of BAG3 in dilated cardiomyopathy and its association with Charcot-Marie-Tooth disease type 2.Acta Myol. 2022 Jun 30;41(2):59-75. doi: 10.36185/2532-1900-071. eCollection 2022 Jun. Acta Myol. 2022. PMID: 35832504 Free PMC article. Review.
Cited by
-
Cardiac fibroblasts: answering the call.Am J Physiol Heart Circ Physiol. 2024 Sep 1;327(3):H681-H686. doi: 10.1152/ajpheart.00478.2024. Epub 2024 Aug 2. Am J Physiol Heart Circ Physiol. 2024. PMID: 39093000 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

