The emergence of human gastrulation upon in vitro attachment
- PMID: 38101401
- PMCID: PMC10828709
- DOI: 10.1016/j.stemcr.2023.11.005
The emergence of human gastrulation upon in vitro attachment
Abstract
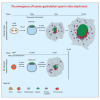
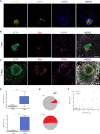
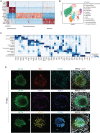
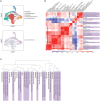
While studied extensively in model systems, human gastrulation remains obscure. The scarcity of fetal biological material as well as ethical considerations limit our understanding of this process. In vitro attachment of natural blastocysts shed light on aspects of the second week of human development in the absence of the morphological manifestation of gastrulation. Stem cell-derived blastocyst models, blastoids, provide the opportunity to reconstitute pre- to post-implantation development in vitro. Here we show that upon in vitro attachment, human blastoids self-organize a BRA+ population and undergo gastrulation. Single-cell RNA sequencing of these models replicates the transcriptomic signature of the human gastrula. Analysis of developmental timing reveals that in both blastoid models and natural human embryos, the onset of gastrulation as defined by molecular markers, can be traced to timescales equivalent to 12 days post fertilization. In all, natural human embryos and blastoid models self-organize primitive streak and mesoderm derivatives upon in vitro attachment.
Keywords: blastoids; gastrulation; human embryo; in vitro attachment; scRNA-seq.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.H.B. is the co-founder of RUMI Scientific and OvaNova. A.H.B. and E.A.R.-O. are shareholders of RUMI Scientific.
Figures
Similar articles
-
3D-cultured blastoids model human embryogenesis from pre-implantation to early gastrulation stages.Cell Stem Cell. 2023 Sep 7;30(9):1148-1165.e7. doi: 10.1016/j.stem.2023.08.005. Cell Stem Cell. 2023. PMID: 37683602
-
Cynomolgus monkey embryo model captures gastrulation and early pregnancy.Cell Stem Cell. 2023 Apr 6;30(4):362-377.e7. doi: 10.1016/j.stem.2023.03.009. Cell Stem Cell. 2023. PMID: 37028403
-
In vitro attachment and symmetry breaking of a human embryo model assembled from primed embryonic stem cells.Cell Stem Cell. 2022 Jun 2;29(6):962-972.e4. doi: 10.1016/j.stem.2022.05.001. Cell Stem Cell. 2022. PMID: 35659878
-
Generating human blastoids modeling blastocyst-stage embryos and implantation.Nat Protoc. 2023 May;18(5):1584-1620. doi: 10.1038/s41596-023-00802-1. Epub 2023 Feb 15. Nat Protoc. 2023. PMID: 36792779 Review.
-
Signaling mechanisms that direct cell fate specification and morphogenesis in human embryonic stem cells-based models of human gastrulation.Emerg Top Life Sci. 2023 Dec 18;7(4):383-396. doi: 10.1042/ETLS20230084. Emerg Top Life Sci. 2023. PMID: 38087898 Review.
References
-
- Arnold S.J., Robertson E.J. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Bio. 2009;10:91–103. - PubMed
-
- Berg S., Kutra D., Kroeger T., Straehle C.N., Kausler B.X., Haubold C., Schiegg M., Ales J., Beier T., Rudy M., et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods. 2019;16:1226–1232. - PubMed
-
- Deglincerti A., Croft G.F., Pietila L.N., Zernicka-Goetz M., Siggia E.D., Brivanlou A.H. Self-organization of the in vitro attached human embryo. Nature. 2016;533:251–254. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

