Broadening the horizon: potential applications of CAR-T cells beyond current indications
- PMID: 38090582
- PMCID: PMC10711079
- DOI: 10.3389/fimmu.2023.1285406
Broadening the horizon: potential applications of CAR-T cells beyond current indications
Abstract
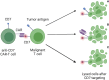
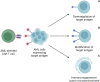
Engineering immune cells to treat hematological malignancies has been a major focus of research since the first resounding successes of CAR-T-cell therapies in B-ALL. Several diseases can now be treated in highly therapy-refractory or relapsed conditions. Currently, a number of CD19- or BCMA-specific CAR-T-cell therapies are approved for acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), multiple myeloma (MM), and follicular lymphoma (FL). The implementation of these therapies has significantly improved patient outcome and survival even in cases with previously very poor prognosis. In this comprehensive review, we present the current state of research, recent innovations, and the applications of CAR-T-cell therapy in a selected group of hematologic malignancies. We focus on B- and T-cell malignancies, including the entities of cutaneous and peripheral T-cell lymphoma (T-ALL, PTCL, CTCL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), chronic lymphocytic leukemia (CLL), classical Hodgkin-Lymphoma (HL), Burkitt-Lymphoma (BL), hairy cell leukemia (HCL), and Waldenström's macroglobulinemia (WM). While these diseases are highly heterogenous, we highlight several similarly used approaches (combination with established therapeutics, target depletion on healthy cells), targets used in multiple diseases (CD30, CD38, TRBC1/2), and unique features that require individualized approaches. Furthermore, we focus on current limitations of CAR-T-cell therapy in individual diseases and entities such as immunocompromising tumor microenvironment (TME), risk of on-target-off-tumor effects, and differences in the occurrence of adverse events. Finally, we present an outlook into novel innovations in CAR-T-cell engineering like the use of artificial intelligence and the future role of CAR-T cells in therapy regimens in everyday clinical practice.
Keywords: AML; CAR-T-cell therapy; CLL; CML; T-cell malignancies; Waldenström’s macroglobulinemia; hairy cell leukemia; lymphoma.
Copyright © 2023 Karsten, Matrisch, Cichutek, Fiedler, Alsdorf and Block.
Conflict of interest statement
WA has received honoraria from GSK and Janssen, payment of expenses by Janssen and Biontech and research funding received by institution for clinical research by Affimed and Biontech. WF participated in advisory boards for Morphosys, AbbVie, Pfizer, Amgen, Jazz Pharmaceuticals, and Clinigen, received support for meeting attendance from Amgen, Jazz Pharmaceuticals, Daiichi Sankyo Oncology, Bristol Myers Squibb, and Servier, received support for medical writing from Amgen, Boehringer Ingelheim, Pfizer, and AbbVie, and received research funding from Apis Technologies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
-
Chimeric antigen receptor T (CAR-T) cells: Novel cell therapy for hematological malignancies.Cancer Med. 2023 Apr;12(7):7844-7858. doi: 10.1002/cam4.5551. Epub 2022 Dec 30. Cancer Med. 2023. PMID: 36583504 Free PMC article. Review.
-
Application of Chimeric Antigen Receptor T Cells in the Treatment of Hematological Malignancies.Biomed Res Int. 2020 Oct 1;2020:4241864. doi: 10.1155/2020/4241864. eCollection 2020. Biomed Res Int. 2020. PMID: 33062678 Free PMC article. Review.
-
Paediatric Strategy Forum for medicinal product development of chimeric antigen receptor T-cells in children and adolescents with cancer: ACCELERATE in collaboration with the European Medicines Agency with participation of the Food and Drug Administration.Eur J Cancer. 2022 Jan;160:112-133. doi: 10.1016/j.ejca.2021.10.016. Epub 2021 Nov 25. Eur J Cancer. 2022. PMID: 34840026 Review.
-
New targets for CAR T therapy in hematologic malignancies.Best Pract Res Clin Haematol. 2021 Sep;34(3):101277. doi: 10.1016/j.beha.2021.101277. Epub 2021 Jun 9. Best Pract Res Clin Haematol. 2021. PMID: 34625226 Review.
Cited by
-
Use of Eltrombopag to Improve Thrombocytopenia and Tranfusion Requirement in Anti-CD19 CAR-T Cell-Treated Patients.J Clin Med. 2024 Aug 28;13(17):5117. doi: 10.3390/jcm13175117. J Clin Med. 2024. PMID: 39274330 Free PMC article.
-
CAR-NKT Cells in Asthma: Use of NKT as a Promising Cell for CAR Therapy.Clin Rev Allergy Immunol. 2024 Jun;66(3):328-362. doi: 10.1007/s12016-024-08998-0. Epub 2024 Jul 12. Clin Rev Allergy Immunol. 2024. PMID: 38995478 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

