Development of LIBRA-seq for the guinea pig model system as a tool for the evaluation of antibody responses to multivalent HIV-1 vaccines
- PMID: 38085509
- PMCID: PMC10804973
- DOI: 10.1128/jvi.01478-23
Development of LIBRA-seq for the guinea pig model system as a tool for the evaluation of antibody responses to multivalent HIV-1 vaccines
Abstract
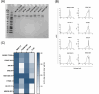
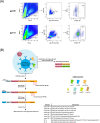
Consistent elicitation of serum antibody responses that neutralize diverse clades of HIV-1 remains a primary goal of HIV-1 vaccine research. Prior work has defined key features of soluble HIV-1 Envelope (Env) immunogen cocktails that influence the neutralization breadth and potency of multivalent vaccine-elicited antibody responses including the number of Env strains in the regimen. We designed immunization groups that consisted of different numbers of SOSIP Env strains to be used in a cocktail immunization strategy: the smallest cocktail (group 2) consisted of a set of two Env strains, which were a subset of the three Env strains that made up group 3, which, in turn, were a subset of the six Env strains that made up group 4. Serum neutralizing titers were modestly broader in guinea pigs that were immunized with a cocktail of three Envs compared to cocktails of two and six, suggesting that multivalent Env immunization could provide a benefit but may be detrimental when the cocktail size is too large. We then adapted the LIBRA-seq platform for antibody discovery to be compatible with guinea pigs, and isolated several tier 2 neutralizing monoclonal antibodies. Three antibodies isolated from two separate guinea pigs were similar in their gene usage and CDR3s, establishing evidence for a guinea pig public clonotype elicited through vaccination. Taken together, this work investigated multivalent HIV-1 Env immunization strategies and provides a novel methodology for screening guinea pig B cell receptor antigen specificity at a high-throughput level using LIBRA-seq.IMPORTANCEMultivalent vaccination with soluble Env immunogens is at the forefront of HIV-1 vaccination strategies but little is known about the influence of the number of Env strains included in vaccine cocktails. Our results suggest that adding more strains is sometimes beneficial but may be detrimental when the number of strains is too high. In addition, we adapted the LIBRA-seq platform to be compatible with guinea pig samples and isolated several tier 2 neutralizing monoclonal antibodies, some of which share V and J gene usage and >70% CDR3 identity, thus establishing the existence of public clonotypes in guinea pigs elicited through vaccination.
Keywords: HIV-1; LIBRA-seq; guinea pig; immunogen; monoclonal antibodies; vaccine.
Conflict of interest statement
M.J.V. and I.S.G. are listed as inventors on patents filed describing the antibodies discovered here. I.S.G. is listed as an inventor on the patent applications for the LIBRA-seq technology. I.S.G. is a co-founder of AbSeek Bio. I.S.G. has served as a consultant for Sanofi. The Georgiev laboratory at VUMC has received unrelated funding from Merck and Takeda Pharmaceuticals.
Figures





Similar articles
-
Elicitation of Neutralizing Antibody Responses to HIV-1 Immunization with Nanoparticle Vaccine Platforms.Viruses. 2021 Jul 2;13(7):1296. doi: 10.3390/v13071296. Viruses. 2021. PMID: 34372503 Free PMC article.
-
Rapid Induction of Multifunctional Antibodies in Rabbits and Macaques by Clade C HIV-1 CAP257 Envelopes Circulating During Epitope-Specific Neutralization Breadth Development.Front Immunol. 2020 Jun 2;11:984. doi: 10.3389/fimmu.2020.00984. eCollection 2020. Front Immunol. 2020. PMID: 32582155 Free PMC article.
-
HIV-1 Cross-Reactive Primary Virus Neutralizing Antibody Response Elicited by Immunization in Nonhuman Primates.J Virol. 2017 Oct 13;91(21):e00910-17. doi: 10.1128/JVI.00910-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28835491 Free PMC article.
-
'Immunization during ART and ATI for HIV-1 vaccine discovery/development'.Curr Opin HIV AIDS. 2023 Nov 1;18(6):309-314. doi: 10.1097/COH.0000000000000817. Epub 2023 Sep 8. Curr Opin HIV AIDS. 2023. PMID: 37712859 Free PMC article. Review.
-
Strategies for inducing effective neutralizing antibody responses against HIV-1.Expert Rev Vaccines. 2019 Nov;18(11):1127-1143. doi: 10.1080/14760584.2019.1690458. Epub 2019 Dec 2. Expert Rev Vaccines. 2019. PMID: 31791150 Free PMC article. Review.
References
-
- Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, Katinger H, Parren PWIH, Robinson J, Van Ryk D, Wang L, Burton DR, Freire E, Wyatt R, Sodroski J, Hendrickson WA, Arthos J. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678–682. doi:10.1038/nature01188 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

