Proximal tubule cells in blood and urine as potential biomarkers for kidney disease biopsy
- PMID: 38077419
- PMCID: PMC10710128
- DOI: 10.7717/peerj.16499
Proximal tubule cells in blood and urine as potential biomarkers for kidney disease biopsy
Abstract
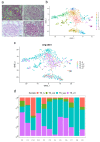
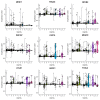
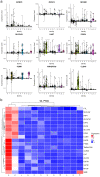
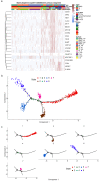
Early diagnosis and treatment are crucial for managing kidney disease, yet there remains a need to further explore pathological mechanisms and develop minimally invasive diagnostic methods. In this study, we employed single-cell RNA sequencing (scRNA-seq) to assess the cellular heterogeneity of kidney diseases. We analyzed gene expression profiles from renal tissue, peripheral blood mononuclear cells (PBMCs), and urine of four patients with nephritis. Our findings identified 12 distinct cell subsets in renal tissues and leukocytes. These subsets encompassed fibroblast cells, mesangial cells, epithelial cells, proximal tubule cells (PTCs), and six immune cell types: CD8+ T cells, macrophages, natural killer cells, dendritic cells, B cells, and neutrophils. Interestingly, PTCs were present in both PBMCs and urine samples but absent in healthy blood samples. Furthermore, several populations of fibroblast cells, mesangial cells, and PTCs exhibited pro-inflammatory or pro-apoptotic behaviors. Our gene expression analysis highlighted the critical role of inflammatory PTCs and fibroblasts in nephritis development and progression. These cells showed high expression of pro-inflammatory genes, which could have chemotactic and activating effect on neutrophils. This was substantiated by the widespread in these cells. Notably, the gene expression profiles of inflammatory PTCs in PBMCs, urine, and kidney tissues had high similarity. This suggests that PTCs in urine and PBMCs hold significant potential as alternative markers to invasive kidney biopsies.
Keywords: Cell exfoliation; Inflammatory proximal tubule cells; Nephritis; Neutropil; Single-cell sequencing.
©2023 Lin et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


Similar articles
-
Deep Phenotyping of Urinary Leukocytes by Mass Cytometry Reveals a Leukocyte Signature for Early and Non-Invasive Prediction of Response to Treatment in Active Lupus Nephritis.Front Immunol. 2020 Mar 24;11:256. doi: 10.3389/fimmu.2020.00256. eCollection 2020. Front Immunol. 2020. PMID: 32265898 Free PMC article.
-
Single-Nucleus RNA Sequencing Identifies New Classes of Proximal Tubular Epithelial Cells in Kidney Fibrosis.J Am Soc Nephrol. 2021 Oct;32(10):2501-2516. doi: 10.1681/ASN.2020081143. Epub 2021 Jun 21. J Am Soc Nephrol. 2021. PMID: 34155061 Free PMC article.
-
A single-cell survey of the human glomerulonephritis.J Cell Mol Med. 2021 May;25(10):4684-4695. doi: 10.1111/jcmm.16407. Epub 2021 Mar 22. J Cell Mol Med. 2021. PMID: 33754492 Free PMC article.
-
Heterogeneity of immune cells in human atherosclerosis revealed by scRNA-Seq.Cardiovasc Res. 2021 Nov 22;117(13):2537-2543. doi: 10.1093/cvr/cvab260. Cardiovasc Res. 2021. PMID: 34343272 Free PMC article. Review.
-
The Advances of Single-Cell RNA-Seq in Kidney Immunology.Front Physiol. 2021 Oct 13;12:752679. doi: 10.3389/fphys.2021.752679. eCollection 2021. Front Physiol. 2021. PMID: 34721077 Free PMC article. Review.
Cited by
-
Associations of the Serum KL-6 with Severity and Prognosis in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease.Lung. 2024 Jun;202(3):245-255. doi: 10.1007/s00408-024-00702-5. Epub 2024 May 14. Lung. 2024. PMID: 38743087
References
-
- Arazi A, Rao DA, Berthier CC, Davidson A, Liu Y, Hoover PJ, Chicoine A, Eisenhaure TM, Jonsson AH, Li S, Lieb DJ, Zhang F, Slowikowski K, Browne EP, Noma A, Sutherby D, Steelman S, Smilek DE, Tosta P, Apruzzese W, Massarotti E, Dall’Era M, Park M, Kamen DL, Furie RA, Payan-Schober F, Pendergraft WFR, McInnis EA, Buyon JP, Petri MA, Putterman C, Kalunian KC, Woodle ES, Lederer JA, Hildeman DA, Nusbaum C, Raychaudhuri S, Kretzler M, Anolik JH, Brenner MB, Wofsy D, Hacohen N, Diamond B. The immune cell landscape in kidneys of patients with lupus nephritis. Nature Immunology. 2019;20:902–914. doi: 10.1038/s41590-019-0398-x. - DOI - PMC - PubMed
-
- Bloom K, Mohsen AW, Karunanidhi A, El DD, Reyes-Mugica M, Wang Y, Ghaloul-Gonzalez L, Otsubo C, Tobita K, Muzumdar R, Gong Z, Tas E, Basu S, Chen J, Bennett M, Hoppel C, Vockley J. Investigating the link of ACAD10 deficiency to type 2 diabetes mellitus. Journal of Inherited Metabolic Disease. 2018;41:49–57. doi: 10.1007/s10545-017-0013-y. - DOI - PMC - PubMed
-
- Buscher AK, Buscher R, Pridzun L, Langkamp M, Wachendorfer N, Hoyer PF, Ranke MB, Hauffa BP. Functional and total IGFBP3 for the assessment of disorders of the GH/IGF1 axis in children with chronic kidney disease, GH deficiency, or short stature after SGA status at birth. European Journal of Endocrinology. 2012;166:923–931. doi: 10.1530/EJE-11-0923. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

