Potentiation of natural killer cells to overcome cancer resistance to NK cell-based therapy and to enhance antibody-based immunotherapy
- PMID: 38077389
- PMCID: PMC10704476
- DOI: 10.3389/fimmu.2023.1275904
Potentiation of natural killer cells to overcome cancer resistance to NK cell-based therapy and to enhance antibody-based immunotherapy
Abstract
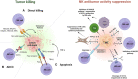
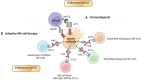
Natural killer (NK) cells are cellular components of the innate immune system that can recognize and suppress the proliferation of cancer cells. NK cells can eliminate cancer cells through direct lysis, by secreting perforin and granzymes, or through antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC involves the binding of the Fc gamma receptor IIIa (CD16), present on NK cells, to the constant region of an antibody already bound to cancer cells. Cancer cells use several mechanisms to evade antitumor activity of NK cells, including the accumulation of inhibitory cytokines, recruitment and expansion of immune suppressor cells such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), modulation of ligands for NK cells receptors. Several strategies have been developed to enhance the antitumor activity of NK cells with the goal of overcoming cancer cells resistance to NK cells. The three main strategies to engineer and boost NK cells cytotoxicity include boosting NK cells with modulatory cytokines, adoptive NK cell therapy, and the employment of engineered NK cells to enhance antibody-based immunotherapy. Although the first two strategies improved the efficacy of NK cell-based therapy, there are still some limitations, including immune-related adverse events, induction of immune-suppressive cells and further cancer resistance to NK cell killing. One strategy to overcome these issues is the combination of monoclonal antibodies (mAbs) that mediate ADCC and engineered NK cells with potentiated anti-cancer activity. The advantage of using mAbs with ADCC activity is that they can activate NK cells, but also favor the accumulation of immune effector cells to the tumor microenvironment (TME). Several clinical trials reported that combining engineered NK cells with mAbs with ADCC activity can result in a superior clinical response compared to mAbs alone. Next generation of clinical trials, employing engineered NK cells with mAbs with higher affinity for CD16 expressed on NK cells, will provide more effective and higher-quality treatments to cancer patients.
Keywords: ADCC; CAR-NK; NK cells; adoptive NK cell therapy; modulatory cytokines; monoclonal antibody.
Copyright © 2023 Fantini, Arlen and Tsang.
Conflict of interest statement
MF, KT and PA are employees of Precision Biologics, Inc. PA has an ownership interest in Precision Biologics, Inc. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Bispecific killer cell engager with high affinity and specificity toward CD16a on NK cells for cancer immunotherapy.Front Immunol. 2023 Jan 6;13:1039969. doi: 10.3389/fimmu.2022.1039969. eCollection 2022. Front Immunol. 2023. PMID: 36685519 Free PMC article.
-
Anti-tumor effects of NK cells and anti-PD-L1 antibody with antibody-dependent cellular cytotoxicity in PD-L1-positive cancer cell lines.J Immunother Cancer. 2020 Aug;8(2):e000873. doi: 10.1136/jitc-2020-000873. J Immunother Cancer. 2020. PMID: 32830112 Free PMC article.
-
A potential therapy for chordoma via antibody-dependent cell-mediated cytotoxicity employing NK or high-affinity NK cells in combination with cetuximab.J Neurosurg. 2018 May;128(5):1419-1427. doi: 10.3171/2017.1.JNS162610. Epub 2017 Jul 28. J Neurosurg. 2018. PMID: 28753113 Free PMC article.
-
Antibody-Dependent Cell-Mediated Cytotoxicity Through Natural Killer (NK) Cells: Unlocking NK Cells for Future Immunotherapy.Curr Pharm Biotechnol. 2022;23(4):552-578. doi: 10.2174/1389201022666210820093608. Curr Pharm Biotechnol. 2022. PMID: 34414871 Review.
-
Antibody-dependent cell cytotoxicity: immunotherapy strategies enhancing effector NK cells.Immunol Cell Biol. 2017 Apr;95(4):347-355. doi: 10.1038/icb.2017.6. Epub 2017 Feb 21. Immunol Cell Biol. 2017. PMID: 28138156 Review.
Cited by
-
Changes in subset distribution and impaired function of circulating natural killer cells in patients with colorectal cancer.Sci Rep. 2024 May 28;14(1):12188. doi: 10.1038/s41598-024-63103-x. Sci Rep. 2024. PMID: 38806640 Free PMC article.
-
NF-κB signaling pathway in tumor microenvironment.Front Immunol. 2024 Oct 18;15:1476030. doi: 10.3389/fimmu.2024.1476030. eCollection 2024. Front Immunol. 2024. PMID: 39493763 Free PMC article. Review.
-
Approaches and challenges in cancer immunotherapy pathways.World J Clin Oncol. 2024 Mar 24;15(3):378-380. doi: 10.5306/wjco.v15.i3.378. World J Clin Oncol. 2024. PMID: 38576600 Free PMC article.
-
Natural killer cells in neuroblastoma: immunological insights and therapeutic perspectives.Cancer Metastasis Rev. 2024 Dec;43(4):1401-1417. doi: 10.1007/s10555-024-10212-8. Epub 2024 Sep 18. Cancer Metastasis Rev. 2024. PMID: 39294470 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

