Autophagy-mediated NKG2D internalization impairs NK cell function and exacerbates radiation pneumonitis
- PMID: 38077388
- PMCID: PMC10704197
- DOI: 10.3389/fimmu.2023.1250920
Autophagy-mediated NKG2D internalization impairs NK cell function and exacerbates radiation pneumonitis
Abstract
Introduction: Radiation pneumonitis is a critical complication that constrains the use of radiation therapy for thoracic malignancies, leading to substantial morbidity via respiratory distress and lung function impairment. The role of Natural killer (NK) cells in inflammatory diseases is well-documented; however, their involvement in radiation pneumonitis is not fully understood.
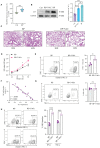
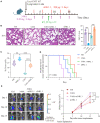
Methods: To explore the involvement of NK cells in radiation pneumonitis, we analyzed tissue samples for NK cell presence and function. The study utilized immunofluorescence staining, western blotting, and immunoprecipitation to investigate CXCL10 and ROS levels, autophagy activity, and NKG2D receptor dynamics in NK cells derived from patients and animal models subjected to radiation.
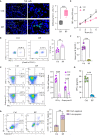
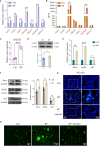
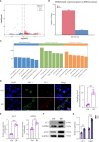
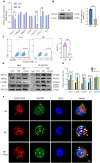
Result: In this study, we observed an augmented infiltration of NK cells in tissues affected by radiation pneumonitis, although their function was markedly diminished. In animal models, enhancing NK cell activity appeared to decelerate the disease progression. Concomitant with the disease course, there was a notable upsurge in CXCL10 and ROS levels. CXCL10 was found to facilitate NK cell migration through CXCR3 receptor activation. Furthermore, evidence of excessive autophagy in patient NK cells was linked to ROS accumulation, as indicated by immunofluorescence and Western blot analyses. The association between the NKG2D receptor and its adaptor proteins (AP2 subunits AP2A1 and AP2M1), LC3, and lysosomes was intensified after radiation exposure, as demonstrated by immunoprecipitation. This interaction led to NKG2D receptor endocytosis and subsequent lysosomal degradation.
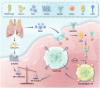
Conclusion: Our findings delineate a mechanism by which radiation-induced lung injury may suppress NK cell function through an autophagy-dependent pathway. The dysregulation observed suggests potential therapeutic targets; hence, modulating autophagy and enhancing NK cell activity could represent novel strategies for mitigating radiation pneumonitis.
Keywords: CXCL10/CXCR3; NK cell; NKG2D; autophagy; radiation pneumonitis.
Copyright © 2023 Wang, Ma, Zhang, Jiang, Mao, Li, Tian and Cheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The traffic of the NKG2D/Dap10 receptor complex during natural killer (NK) cell activation.J Biol Chem. 2009 Jun 12;284(24):16463-16472. doi: 10.1074/jbc.M808561200. Epub 2009 Mar 26. J Biol Chem. 2009. PMID: 19329438 Free PMC article.
-
Enhanced interaction between natural killer cells and lung cancer cells: involvement in gefitinib-mediated immunoregulation.J Transl Med. 2013 Aug 12;11:186. doi: 10.1186/1479-5876-11-186. J Transl Med. 2013. PMID: 23937717 Free PMC article.
-
Bispecific antibody-mediated redirection of NKG2D-CAR natural killer cells facilitates dual targeting and enhances antitumor activity.J Immunother Cancer. 2021 Oct;9(10):e002980. doi: 10.1136/jitc-2021-002980. J Immunother Cancer. 2021. PMID: 34599028 Free PMC article.
-
Regulation of NKG2D-Dependent NK Cell Functions: The Yin and the Yang of Receptor Endocytosis.Int J Mol Sci. 2017 Aug 2;18(8):1677. doi: 10.3390/ijms18081677. Int J Mol Sci. 2017. PMID: 28767057 Free PMC article. Review.
-
Role of Polymorphisms of NKG2D Receptor and Its Ligands in Acute Myeloid Leukemia and Human Stem Cell Transplantation.Front Immunol. 2021 Mar 30;12:651751. doi: 10.3389/fimmu.2021.651751. eCollection 2021. Front Immunol. 2021. PMID: 33868289 Free PMC article. Review.
Cited by
-
Advances in the prerequisite and consequence of STING downstream signalosomes.Med Rev (2021). 2024 May 8;4(5):435-451. doi: 10.1515/mr-2024-0016. eCollection 2024 Oct. Med Rev (2021). 2024. PMID: 39444795 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

