Involvement of interferon gamma signaling in spinal trigeminal caudal subnucleus astrocyte in orofacial neuropathic pain in rats with infraorbital nerve injury
- PMID: 38073236
- PMCID: PMC10748561
- DOI: 10.1177/17448069231222403
Involvement of interferon gamma signaling in spinal trigeminal caudal subnucleus astrocyte in orofacial neuropathic pain in rats with infraorbital nerve injury
Abstract
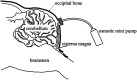
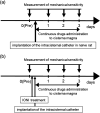
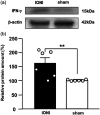
Background: Trigeminal nerve injury causes orofacial pain that can interfere with activities of daily life. However, the underlying mechanism remains unknown, and the appropriate treatment has not been established yet. This study aimed to examine the involvement of interferon gamma (IFN-γ) signaling in the spinal trigeminal caudal subnucleus (Vc) in orofacial neuropathic pain. Methods: Infraorbital nerve (ION) injury (IONI) was performed in rats by partial ION ligation. The head-withdrawal reflex threshold (HWT) to mechanical stimulation of the whisker pad skin was measured in IONI or sham rats, as well as following a continuous intracisterna magna administration of IFN-γ and a mixture of IFN-γ and fluorocitrate (inhibitor of astrocytes activation) in naïve rats, or an IFN-γ antagonist in IONI rats. The IFN-γ receptor immunohistochemistry and IFN-γ Western blotting were analyzed in the Vc after IONI or sham treatment. The glial fibrillary acid protein (GFAP) immunohistochemistry and Western blotting were also analyzed after administration of IFN-γ and the mixture of IFN-γ and fluorocitrate. Moreover, the change in single neuronal activity in the Vc was examined in the IONI, sham, and IONI group administered IFN-γ antagonist. Results: The HWT decreased after IONI. The IFN-γ and IFN-γ receptor were upregulated after IONI, and the IFN-γ receptor was expressed in Vc astrocytes. IFN-γ administration decreased the HWT, whereas the mixture of IFN-γ and fluorocitrate recovered the decrement of HWT. IFN-γ administration upregulated GFAP expression, while the mixture of IFN-γ and fluorocitrate recovered the upregulation of GFAP expression. IONI significantly enhanced the neuronal activity of the mechanical-evoked responses, and administration of an IFN-γ antagonist significantly inhibited these enhancements. Conclusions: IFN-γ signaling through the receptor in astrocytes is a key mechanism underlying orofacial neuropathic pain associated with trigeminal nerve injury. These findings will aid in the development of therapeutics for orofacial neuropathic pain.
Keywords: Astrocyte; infraorbital nerve injury; involvement of interferon gamma; orofacial neuropathic pain.
Figures

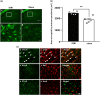

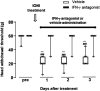
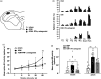
 indicates IFN-γ antagonist-administered IONI group. (b) Typical response of Vc neurons for von Frey filaments (1, 6, 15, 26 and 60 g), brush, and pinch stimulation on whisker pad skin in sham, IONI, and IFN-γ antagonist-administered IONI group. (c) Response to 1, 6, 15, 26 and 60 g pressure stimulation in each group. **: p < 0.01, sham vs. IONI; ††: p < 0.01, IONI vs. IFN-γ antagonist-administered IONI, (Kruskal-Wallis with Dunn’s multiple comparisons tests) (d) Neuronal response to brush, and pinch stimulation on whisker pad skin in sham, IONI, and IFN-γ antagonist-administered IONI group. *: p < 0.05, **: p < 0.01 (one-way ANOVA followed by Sidak multiple comparison test). White circle and bar indicate sham group. Black circle and bar indicate IONI group. Grey triangle and bar indicate IFN-γ antagonist-administered IONI group. BG: background neuronal firing ( 8 WDR and 3 NS neurons from 6 sham rats; 8 WDR and 4 NS neurons from 6 IONI rats; 8 WDR and 3 NS neurons from 6 IFN-γ antagonist-administered IONI).
indicates IFN-γ antagonist-administered IONI group. (b) Typical response of Vc neurons for von Frey filaments (1, 6, 15, 26 and 60 g), brush, and pinch stimulation on whisker pad skin in sham, IONI, and IFN-γ antagonist-administered IONI group. (c) Response to 1, 6, 15, 26 and 60 g pressure stimulation in each group. **: p < 0.01, sham vs. IONI; ††: p < 0.01, IONI vs. IFN-γ antagonist-administered IONI, (Kruskal-Wallis with Dunn’s multiple comparisons tests) (d) Neuronal response to brush, and pinch stimulation on whisker pad skin in sham, IONI, and IFN-γ antagonist-administered IONI group. *: p < 0.05, **: p < 0.01 (one-way ANOVA followed by Sidak multiple comparison test). White circle and bar indicate sham group. Black circle and bar indicate IONI group. Grey triangle and bar indicate IFN-γ antagonist-administered IONI group. BG: background neuronal firing ( 8 WDR and 3 NS neurons from 6 sham rats; 8 WDR and 4 NS neurons from 6 IONI rats; 8 WDR and 3 NS neurons from 6 IFN-γ antagonist-administered IONI).Similar articles
-
Microglia-Astrocyte Communication via C1q Contributes to Orofacial Neuropathic Pain Associated with Infraorbital Nerve Injury.Int J Mol Sci. 2020 Sep 17;21(18):6834. doi: 10.3390/ijms21186834. Int J Mol Sci. 2020. PMID: 32957694 Free PMC article.
-
Increase in IGF-1 Expression in the Injured Infraorbital Nerve and Possible Implications for Orofacial Neuropathic Pain.Int J Mol Sci. 2019 Dec 17;20(24):6360. doi: 10.3390/ijms20246360. Int J Mol Sci. 2019. PMID: 31861182 Free PMC article.
-
Pannexin 1 role in the trigeminal ganglion in infraorbital nerve injury-induced mechanical allodynia.Oral Dis. 2023 May;29(4):1770-1781. doi: 10.1111/odi.14129. Epub 2022 Jan 23. Oral Dis. 2023. PMID: 35029007
-
Non-neuronal cells act as crucial players in neuropathic orofacial pain.J Oral Biosci. 2024 Sep;66(3):491-495. doi: 10.1016/j.job.2024.07.005. Epub 2024 Jul 18. J Oral Biosci. 2024. PMID: 39032826 Review.
-
Neuron-glia interaction is a key mechanism underlying persistent orofacial pain.J Oral Sci. 2017;59(2):173-175. doi: 10.2334/josnusd.16-0858. J Oral Sci. 2017. PMID: 28637974 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

