Opaganib (ABC294640) Induces Immunogenic Tumor Cell Death and Enhances Checkpoint Antibody Therapy
- PMID: 38069222
- PMCID: PMC10706694
- DOI: 10.3390/ijms242316901
Opaganib (ABC294640) Induces Immunogenic Tumor Cell Death and Enhances Checkpoint Antibody Therapy
Abstract
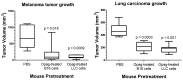
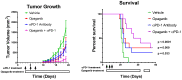
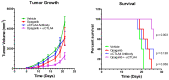
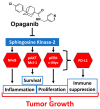
Antibody-based cancer drugs that target the checkpoint proteins CTLA-4, PD-1 and PD-L1 provide marked improvement in some patients with deadly diseases such as lung cancer and melanoma. However, most patients are either unresponsive or relapse following an initial response, underscoring the need for further improvement in immunotherapy. Certain drugs induce immunogenic cell death (ICD) in tumor cells in which the dying cells promote immunologic responses in the host that may enhance the in vivo activity of checkpoint antibodies. Sphingolipid metabolism is a key pathway in cancer biology, in which ceramides and sphingosine 1-phosphate (S1P) regulate tumor cell death, proliferation and drug resistance, as well as host inflammation and immunity. In particular, sphingosine kinases are key sites for manipulation of the ceramide/S1P balance that regulates tumor cell proliferation and sensitivity to radiation and chemotherapy. We and others have demonstrated that inhibition of sphingosine kinase-2 by the small-molecule investigational drug opaganib (formerly ABC294640) kills tumor cells and increases their sensitivities to other drugs and radiation. Because sphingolipids have been shown to regulate ICD, opaganib may induce ICD and improve the efficacy of checkpoint antibodies for cancer therapy. This was demonstrated by showing that in vitro treatment with opaganib increases the surface expression of the ICD marker calreticulin on a variety of tumor cell types. In vivo confirmation was achieved using the gold standard immunization assay in which B16 melanoma, Lewis lung carcinoma (LLC) or Neuro-2a neuroblastoma cells were treated with opaganib in vitro and then injected subcutaneously into syngeneic mice, followed by implantation of untreated tumor cells 7 days later. In all cases, immunization with opaganib-treated cells strongly suppressed the growth of subsequently injected tumor cells. Interestingly, opaganib treatment induced crossover immunity in that opaganib-treated B16 cells suppressed the growth of both untreated B16 and LLC cells and opaganib-treated LLC cells inhibited the growth of both untreated LLC and B16 cells. Next, the effects of opaganib in combination with a checkpoint antibody on tumor growth in vivo were assessed. Opaganib and anti-PD-1 antibody each slowed the growth of B16 tumors and improved mouse survival, while the combination of opaganib plus anti-PD-1 strongly suppressed tumor growth and improved survival (p < 0.0001). Individually, opaganib and anti-CTLA-4 antibody had modest effects on the growth of LLC tumors and mouse survival, whereas the combination of opaganib with anti-CTLA-4 substantially inhibited tumor growth and increased survival (p < 0.001). Finally, the survival of mice bearing B16 tumors was only marginally improved by opaganib or anti-PD-L1 antibody alone but was nearly doubled by the drugs in combination (p < 0.005). Overall, these studies demonstrate the ability of opaganib to induce ICD in tumor cells, which improves the antitumor activity of checkpoint antibodies.
Keywords: ABC294640; checkpoint antibody; immunogenic cell death; immunotherapy; opaganib; sphingolipid.
Conflict of interest statement
Lynn W. Maines, Staci N. Keller and Charles D. Smith are employees of and own stock in Apogee Biotechnology Corporation. Apogee Biotechnology Corporation owns patent rights to opaganib, and the value of these rights may be affected by the research reported in the enclosed paper. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Opaganib Downregulates N-Myc Expression and Suppresses In Vitro and In Vivo Growth of Neuroblastoma Cells.Cancers (Basel). 2024 May 5;16(9):1779. doi: 10.3390/cancers16091779. Cancers (Basel). 2024. PMID: 38730731 Free PMC article.
-
Opaganib Protects against Radiation Toxicity: Implications for Homeland Security and Antitumor Radiotherapy.Int J Mol Sci. 2022 Oct 29;23(21):13191. doi: 10.3390/ijms232113191. Int J Mol Sci. 2022. PMID: 36361977 Free PMC article.
-
The Sphingolipid-Modulating Drug Opaganib Protects against Radiation-Induced Lung Inflammation and Fibrosis: Potential Uses as a Medical Countermeasure and in Cancer Radiotherapy.Int J Mol Sci. 2024 Feb 15;25(4):2322. doi: 10.3390/ijms25042322. Int J Mol Sci. 2024. PMID: 38396999 Free PMC article.
-
Recent Progress in the Development of Opaganib for the Treatment of Covid-19.Drug Des Devel Ther. 2022 Jul 12;16:2199-2211. doi: 10.2147/DDDT.S367612. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 35855741 Free PMC article. Review.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
Cited by
-
Proteomics and lipidomic analysis reveal dysregulated pathways associated with loss of sacsin.Front Neurosci. 2024 Jun 7;18:1375299. doi: 10.3389/fnins.2024.1375299. eCollection 2024. Front Neurosci. 2024. PMID: 38911600 Free PMC article.
-
Opaganib Downregulates N-Myc Expression and Suppresses In Vitro and In Vivo Growth of Neuroblastoma Cells.Cancers (Basel). 2024 May 5;16(9):1779. doi: 10.3390/cancers16091779. Cancers (Basel). 2024. PMID: 38730731 Free PMC article.
References
-
- Robert C., Ribas A., Hamid O., Daud A., Wolchok J.D., Joshua A.M., Hwu W.J., Weber J.S., Gangadhar T.C., Joseph R.W., et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018;36:1668–1674. doi: 10.1200/JCO.2017.75.6270. - DOI - PubMed
-
- Meybodi S.M., Farasati Far B., Pourmolaei A., Baradarbarjastehbaf F., Safaei M., Mohammadkhani N., Samadani A.A. Immune checkpoint inhibitors promising role in cancer therapy: Clinical evidence and immune-related adverse events. Med. Oncol. 2023;40:243. doi: 10.1007/s12032-023-02114-6. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

