The Fundamental Role of Oxime and Oxime Ether Moieties in Improving the Physicochemical and Anticancer Properties of Structurally Diverse Scaffolds
- PMID: 38069175
- PMCID: PMC10705934
- DOI: 10.3390/ijms242316854
The Fundamental Role of Oxime and Oxime Ether Moieties in Improving the Physicochemical and Anticancer Properties of Structurally Diverse Scaffolds
Abstract
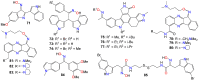
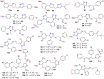
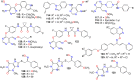
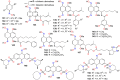
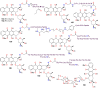
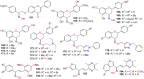
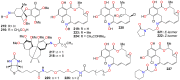
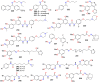
The present review explores the critical role of oxime and oxime ether moieties in enhancing the physicochemical and anticancer properties of structurally diverse molecular frameworks. Specific examples are carefully selected to illustrate the distinct contributions of these functional groups to general strategies for molecular design, modulation of biological activities, computational modeling, and structure-activity relationship studies. An extensive literature search was conducted across three databases, including PubMed, Google Scholar, and Scifinder, enabling us to create one of the most comprehensive overviews of how oximes and oxime ethers impact antitumor activities within a wide range of structural frameworks. This search focused on various combinations of keywords or their synonyms, related to the anticancer activity of oximes and oxime ethers, structure-activity relationships, mechanism of action, as well as molecular dynamics and docking studies. Each article was evaluated based on its scientific merit and the depth of the study, resulting in 268 cited references and more than 336 illustrative chemical structures carefully selected to support this analysis. As many previous reviews focus on one subclass of this extensive family of compounds, this report represents one of the rare and fully comprehensive assessments of the anticancer potential of this group of molecules across diverse molecular scaffolds.
Keywords: SAR; anticancer activities; docking studies; oxime ethers; oximes; physicochemical properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Steroidal Oximes: Useful Compounds with Antitumor Activities.Curr Med Chem. 2018 Feb 21;25(6):660-686. doi: 10.2174/0929867324666171003115400. Curr Med Chem. 2018. PMID: 28971759 Review.
-
Design, synthesis, and biological activity of oxime ether strobilurin derivatives containing indole moiety as novel fungicide.Chem Biol Drug Des. 2015 Jun;85(6):743-55. doi: 10.1111/cbdd.12460. Epub 2014 Nov 15. Chem Biol Drug Des. 2015. PMID: 25346294
-
Design, Synthesis, and Insecticidal and Fungicidal Activities of Ether/Oxime-ether Containing Isoxazoline Derivatives.J Agric Food Chem. 2023 Apr 5;71(13):5107-5116. doi: 10.1021/acs.jafc.2c08161. Epub 2023 Mar 22. J Agric Food Chem. 2023. PMID: 36947168
-
Nitrogen-containing ecdysteroid derivatives vs. multi-drug resistance in cancer: Preparation and antitumor activity of oximes, oxime ethers and a lactam.Eur J Med Chem. 2018 Jan 20;144:730-739. doi: 10.1016/j.ejmech.2017.12.032. Epub 2017 Dec 12. Eur J Med Chem. 2018. PMID: 29291440
-
A Review of Biologically Active Oxime Ethers.Molecules. 2023 Jun 28;28(13):5041. doi: 10.3390/molecules28135041. Molecules. 2023. PMID: 37446703 Free PMC article. Review.
Cited by
-
Editorial for the Special Issue "Advances in the Development of Anticancer Drugs".Int J Mol Sci. 2024 Jan 4;25(1):641. doi: 10.3390/ijms25010641. Int J Mol Sci. 2024. PMID: 38203815 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

