Folate-Appended Hydroxypropyl-β-Cyclodextrin Induces Autophagic Cell Death in Acute Myeloid Leukemia Cells
- PMID: 38069042
- PMCID: PMC10706821
- DOI: 10.3390/ijms242316720
Folate-Appended Hydroxypropyl-β-Cyclodextrin Induces Autophagic Cell Death in Acute Myeloid Leukemia Cells
Abstract
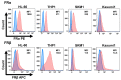
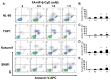
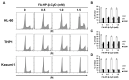
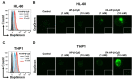
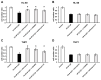
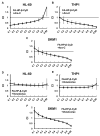
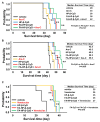
Acute myeloid leukemia (AML) is a heterogenous myeloid neoplasm that remains challenging to treat. Because intensive conventional chemotherapy reduces survival rates in elderly patients, drugs with lower toxicity and fewer side effects are needed urgently. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CyD) is used clinically as a pharmaceutical excipient for poorly water-soluble drugs. Previously, we showed that HP-β-CyD exerts antitumor activity by disrupting cholesterol homeostasis. Recently, we developed folate-conjugated HP-β-CyD (FA-HP-β-CyD) and demonstrated its potential as a new antitumor agent that induces not only apoptosis, but also autophagic cell death; however, we do not know whether FA-HP-β-CyD exerts these effects against AML. Here, we investigated the effects of FA-HP-β-CyD on folate receptor (FR)-expressing AML cells. We found that the cytotoxic activity of FA-HP-β-CyD against AML cells was stronger than that of HP-β-CyD. Also, FA-HP-CyD induced the formation of autophagosomes in AML cell lines. FA-HP-β-CyD increased the inhibitory effects of cytarabine and a BCL-2-selective inhibitor, Venetoclax, which are commonly used treat elderly AML patients. Notably, FA-HP-β-CyD suppressed the proliferation of AML cells in BALB/c nude recombinase-activating gene-2 (Rag-2)/Janus kinase 3 (Jak3) double-deficient mice with AML. These results suggest that FA-HP-β-CyD acts as a potent anticancer agent for AML chemotherapy by regulating autophagy.
Keywords: 2-hydroxypropyl-β-cyclodextrin; Venetoclax; acute myeloid leukemia; autophagy; cholesterol; folate receptor; folic acid; metabolism; mitochondria; molecular targeting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Evaluation of antitumor effects of folate-conjugated methyl-β-cyclodextrin in melanoma.Biol Pharm Bull. 2015;38(3):374-9. doi: 10.1248/bpb.b14-00531. Biol Pharm Bull. 2015. PMID: 25757918
-
Folic Acid-Appended Hydroxypropyl-β-Cyclodextrin Exhibits Potent Antitumor Activity in Chronic Myeloid Leukemia Cells via Autophagic Cell Death.Cancers (Basel). 2021 Oct 28;13(21):5413. doi: 10.3390/cancers13215413. Cancers (Basel). 2021. PMID: 34771576 Free PMC article.
-
2-Hydroxypropyl-β-Cyclodextrin Acts as a Novel Anticancer Agent.PLoS One. 2015 Nov 4;10(11):e0141946. doi: 10.1371/journal.pone.0141946. eCollection 2015. PLoS One. 2015. PMID: 26535909 Free PMC article.
-
[Development of Cyclodextrin-based Cancer Treatment].Yakugaku Zasshi. 2015;135(11):1291-8. doi: 10.1248/yakushi.15-00202. Yakugaku Zasshi. 2015. PMID: 26521878 Review. Japanese.
-
Sugar-appended polyamidoamine dendrimer conjugates with cyclodextrins as cell-specific non-viral vectors.Adv Drug Deliv Rev. 2013 Aug;65(9):1204-14. doi: 10.1016/j.addr.2013.04.001. Epub 2013 Apr 17. Adv Drug Deliv Rev. 2013. PMID: 23602906 Review.
References
-
- Ohtake S., Miyawaki S., Fujita H., Kiyoi H., Shinagawa K., Usui N., Okumura H., Miyamura K., Nakaseko C., Miyazaki Y., et al. Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: The JALSG AML201 Study. Blood. 2011;117:2358–2365. doi: 10.1182/blood-2010-03-273243. - DOI - PubMed
-
- DiNardo C.D., Pratz K., Pullarkat V., Jonas B.A., Arellano M., Becker P.S., Frankfurt O., Konopleva M., Wei A.H., Kantarjian H.M., et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17. doi: 10.1182/blood-2018-08-868752. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

