Genetic Deficiency of the Long Pentraxin 3 Affects Osteogenesis and Osteoclastogenesis in Homeostatic and Inflammatory Conditions
- PMID: 38068970
- PMCID: PMC10706359
- DOI: 10.3390/ijms242316648
Genetic Deficiency of the Long Pentraxin 3 Affects Osteogenesis and Osteoclastogenesis in Homeostatic and Inflammatory Conditions
Abstract
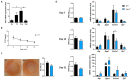
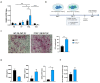
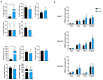
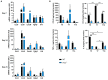
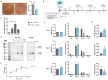
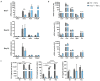
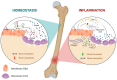
The long pentraxin 3 (PTX3) is a soluble glycoprotein made by immune and nonimmune cells endowed with pleiotropic functions in innate immunity, inflammation, and tissue remodeling. PTX3 has recently emerged as a mediator of bone turnover in both physiological and pathological conditions, with direct and indirect effects on osteoblasts and osteoclasts. This notwithstanding, its role in bone biology, with major regard to the osteogenic potential of osteoblasts and their interplay with osteoclasts, is at present unclear. Here, we investigated the contribution of this pentraxin to bone deposition in the osteogenic lineage by assessing collagen production, mineralization capacity, osteoblast maturation, extracellular matrix gene expression, and inflammatory mediators' production in primary osteoblasts from the calvaria of wild-type (WT) and Ptx3-deficient (Ptx3-/-) mice. Also, we evaluated the effect of PTX3 on osteoclastogenesis in cocultures of primary osteoblasts and bone marrow-derived osteoclasts. Our investigations were carried out both in physiological and inflammatory conditions to recapitulate in vitro aspects of inflammatory diseases of the bone. We found that primary osteoblasts from WT animals constitutively expressed low levels of the protein in osteogenic noninflammatory conditions, and genetic ablation of PTX3 in these cells had no major impact on collagen and hydroxyapatite deposition. However, Ptx3-/- osteoblasts had an increased RANKL/OPG ratio and CD44 expression, which resulted in in enhanced osteoclastogenesis when cocultured with bone marrow monocytes. Inflammation (modelled through administration of tumor necrosis factor-α, TNF-α) boosted the expression and accumulation of PTX3 and inflammatory mediators in WT osteoblasts. In these conditions, Ptx3 genetic depletion was associated with reduced collagen deposition and immune modulators' production. Our study shed light on the role of PTX3 in osteoblast and osteoclast biology and identified a major effect of inflammation on the bone-related properties of this pentraxin, which might be relevant for therapeutic and/or diagnostic purposes in musculoskeletal pathology.
Keywords: PTX3; bone; hyaluronan-rich matrix; osteoblast; osteoclast; tissue remodeling.
Conflict of interest statement
All the authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
PTX3 stimulates osteoclastogenesis by increasing osteoblast RANKL production.J Cell Physiol. 2014 Nov;229(11):1744-52. doi: 10.1002/jcp.24626. J Cell Physiol. 2014. PMID: 24664887
-
Osteoblasts of calvaria induce higher numbers of osteoclasts than osteoblasts from long bone.Bone. 2016 May;86:10-21. doi: 10.1016/j.bone.2016.02.010. Epub 2016 Feb 24. Bone. 2016. PMID: 26921824
-
Resolvin E1 Promotes Bone Preservation Under Inflammatory Conditions.Front Immunol. 2018 Jun 12;9:1300. doi: 10.3389/fimmu.2018.01300. eCollection 2018. Front Immunol. 2018. PMID: 29946319 Free PMC article.
-
The Long Pentraxin PTX3 in Bone Homeostasis and Pathology.Front Immunol. 2019 Nov 8;10:2628. doi: 10.3389/fimmu.2019.02628. eCollection 2019. Front Immunol. 2019. PMID: 31787987 Free PMC article. Review.
-
Osteoclast differentiation by RANKL and OPG signaling pathways.J Bone Miner Metab. 2021 Jan;39(1):19-26. doi: 10.1007/s00774-020-01162-6. Epub 2020 Oct 20. J Bone Miner Metab. 2021. PMID: 33079279 Review.
References
-
- Porte R., Silva-Gomes R., Theroude C., Parente R., Asgari F., Sironi M., Pasqualini F., Valentino S., Asselta R., Recordati C., et al. Regulation of inflammation and protection against invasive pneumococcal infection by the long pentraxin PTX3. eLife. 2023;12:e78601. doi: 10.7554/eLife.78601. - DOI - PMC - PubMed
-
- Noone D.P., Dijkstra D.J., van der Klugt T.T., van Veelen P.A., de Ru A.H., Hensbergen P.J., Trouw L.A., Sharp T.H. PTX3 structure determination using a hybrid cryoelectron microscopy and AlphaFold approach offers insights into ligand binding and complement activation. Proc. Natl. Acad. Sci. USA. 2022;119:e2208144119. doi: 10.1073/pnas.2208144119. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

