Adipocyte Microenvironment in Ovarian Cancer: A Critical Contributor?
- PMID: 38068912
- PMCID: PMC10706733
- DOI: 10.3390/ijms242316589
Adipocyte Microenvironment in Ovarian Cancer: A Critical Contributor?
Abstract
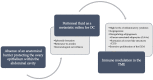
Ovarian cancer is one of the most common gynecological malignancies and has low survival rates. One of the main determinants of this unfavorable prognosis is the high rate of peritoneal metastasis at diagnosis, closely related to its morbidity and mortality. The mechanism underlying peritoneal carcinomatosis is not clearly defined, but a clear preference for omental spread has been described. Growing evidence suggests that adipose tissue plays a role in promoting cancer onset and progression. Moreover, obesity can lead to changes in the original functions of adipocytes, resulting in metabolic and inflammatory changes in the adipose tissue microenvironment, potentially increasing the risk of tumor growth. However, the specific roles of adipocytes in ovarian cancer have not yet been fully elucidated. Due to the undeniable link between obesity and cancer, the adipose tissue microenvironment could also present a promising therapeutic target that warrants further research. This review discusses the complex relationship between ovarian cancer and the adipose tissue microenvironment.
Keywords: adipocyte; ovarian cancer; pathogenesis; peritoneal metastasis; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Adipocyte pyroptosis occurs in omental tumor microenvironment and is associated with chemoresistance of ovarian cancer.J Biomed Sci. 2024 Jun 11;31(1):62. doi: 10.1186/s12929-024-01051-4. J Biomed Sci. 2024. PMID: 38862973 Free PMC article.
-
Adipocytes: active facilitators in epithelial ovarian cancer progression?J Ovarian Res. 2020 Sep 23;13(1):115. doi: 10.1186/s13048-020-00718-4. J Ovarian Res. 2020. PMID: 32967712 Free PMC article. Review.
-
Pro-tumoral behavior of omental adipocyte-derived fibroblasts in tumor microenvironment at the metastatic site of ovarian cancer.Int J Cancer. 2021 Dec 1;149(11):1961-1972. doi: 10.1002/ijc.33770. Epub 2021 Sep 16. Int J Cancer. 2021. PMID: 34469585
-
The adipocyte microenvironment and cancer.Cancer Metastasis Rev. 2022 Sep;41(3):575-587. doi: 10.1007/s10555-022-10059-x. Epub 2022 Aug 8. Cancer Metastasis Rev. 2022. PMID: 35941408 Review.
-
The Tumor Promotional Role of Adipocytes in the Breast Cancer Microenvironment and Macroenvironment.Am J Pathol. 2021 Aug;191(8):1342-1352. doi: 10.1016/j.ajpath.2021.02.006. Epub 2021 Feb 24. Am J Pathol. 2021. PMID: 33639102 Free PMC article. Review.
Cited by
-
Weight loss treatment for COVID-19 in patients with NCDs: a pilot prospective clinical trial.Sci Rep. 2024 May 14;14(1):10979. doi: 10.1038/s41598-024-61703-1. Sci Rep. 2024. PMID: 38744929 Free PMC article. Clinical Trial.
-
Unveiling the causal link between metabolic factors and ovarian cancer risk using Mendelian randomization analysis.Front Endocrinol (Lausanne). 2024 Jun 5;15:1401648. doi: 10.3389/fendo.2024.1401648. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38899007 Free PMC article.
References
-
- Colombo N., Sessa C., du Bois A., Ledermann J., McCluggage W.G., McNeish I., Morice P., Pignata S., Ray-Coquard I., Vergote I., et al. ESMO-ESGO Consensus Conference Recommendations on Ovarian Cancer: Pathology and Molecular Biology, Early and Advanced Stages, Borderline Tumours and Recurrent Disease. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019;30:672–705. doi: 10.1093/annonc/mdz062. - DOI - PubMed
-
- Szender J.B., Emmons T., Belliotti S., Dickson D., Khan A., Morrell K., Khan A.N.M.N., Singel K.L., Mayor P.C., Moysich K.B., et al. Impact of Ascites Volume on Clinical Outcomes in Ovarian Cancer: A Cohort Study. Gynecol. Oncol. 2017;146:491–497. doi: 10.1016/j.ygyno.2017.06.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

