The Multifaceted Roles of Lamins in Lung Cancer and DNA Damage Response
- PMID: 38067205
- PMCID: PMC10705174
- DOI: 10.3390/cancers15235501
The Multifaceted Roles of Lamins in Lung Cancer and DNA Damage Response
Abstract
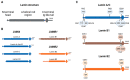
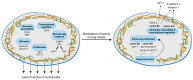
Emerging evidence suggests that lamin functions are not limited to maintaining the structural integrity of the nucleus in eukaryotic cells but that these functions affect many facets of cancer biology. An increasing number of reports suggest that adaptive changes in the lamin subtype composition within the nuclear lamina could affect essential features of cancer development and aggressiveness. These include regulation of cellular stiffness and mobility as well as epithelial-to-mesenchymal transition (EMT), all of which directly impact the metastatic properties of cancer cells. Additionally, insights from studies on the physiological functions of lamins suggest that cancer cells could hijack the ability of lamins to modify chromatin accessibility, cell cycle regulation, and DNA damage response. Here, we present a comprehensive overview of the role of lamins in lung cancer and DNA damage response, which is commonly evoked by lung cancer therapies. Collectively, this information should help better understand the sometimes-conflicting reports on lamin functions in lung cancer as well as in other cancer types.
Keywords: DNA repair; kinases; lamin composition; lamins; lung cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
The wide and growing range of lamin B-related diseases: from laminopathies to cancer.Cell Mol Life Sci. 2022 Feb 8;79(2):126. doi: 10.1007/s00018-021-04084-2. Cell Mol Life Sci. 2022. PMID: 35132494 Free PMC article. Review.
-
Lamins and lamin-binding proteins in functional chromatin organization.Crit Rev Eukaryot Gene Expr. 1999;9(3-4):257-65. doi: 10.1615/critreveukargeneexpr.v9.i3-4.100. Crit Rev Eukaryot Gene Expr. 1999. PMID: 10651242 Review.
-
Emerging roles of lamins and DNA damage repair mechanisms in ovarian cancer.Biochem Soc Trans. 2020 Oct 30;48(5):2317-2333. doi: 10.1042/BST20200713. Biochem Soc Trans. 2020. PMID: 33084906 Review.
-
The dynamic properties and possible functions of nuclear lamins.Int Rev Cytol. 1995;162B:141-82. doi: 10.1016/s0074-7696(08)62616-9. Int Rev Cytol. 1995. PMID: 8557486 Review.
-
Altering lamina assembly reveals lamina-dependent and -independent functions for A-type lamins.J Cell Sci. 2015 Oct 1;128(19):3607-20. doi: 10.1242/jcs.171843. Epub 2015 Aug 14. J Cell Sci. 2015. PMID: 26275827 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

