Role for Nongenomic Estrogen Signaling in Male Fertility
- PMID: 38066676
- PMCID: PMC10797322
- DOI: 10.1210/endocr/bqad180
Role for Nongenomic Estrogen Signaling in Male Fertility
Abstract
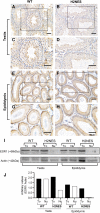
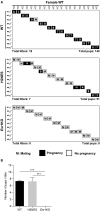
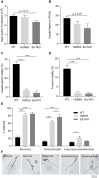
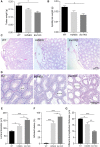
Estrogen actions are mediated by both nuclear (n) and membrane (m) localized estrogen receptor 1 (ESR1). Male Esr1 knockout (Esr1KO) mice lacking functional Esr1 are infertile, with reproductive tract abnormalities. Male mice expressing nESR1 but lacking mESR1 (nuclear-only estrogen receptor 1 mice) are progressively infertile due to testicular, rete testis, and efferent ductule abnormalities similar to Esr1KO males, indicating a role for mESR1 in male reproduction. The H2NES mouse expresses only mESR1 but lacks nESR1. The goal of this study was to identify the functions of mESR1 alone in mice where nESR1 was absent. Breeding trials showed that H2NES males are fertile, with decreased litter numbers but normal pup numbers/litter. In contrast to Esr1KO mice, H2NES testicular, and epididymal weights were not reduced, and seminiferous tubule abnormalities were less pronounced. However, Esr1KO and H2NES males both had decreased sperm motility and a high incidence of abnormal sperm morphology. Seminiferous tubule and rete testis dilation and decreased efferent ductule epithelial height characteristic of Esr1KO males were reduced in H2NES. Consistent with this, expression of genes involved in fluid transport and ion movement that were reduced in Esr1KO (Aqp1, Car2, Car14, Cftr) were partially or fully restored to wild-type levels in H2NES. In summary, in contrast to Esr1KO males, H2NES males are fertile and have reduced phenotypic and functional abnormalities in the testis and efferent ductules. Thus, mESR1 alone, in the absence of nESR1, can partially regulate male reproductive tract structure and function, emphasizing its importance for overall estrogen action.
Keywords: ESR1; efferent ductules; epididymis; estrogen; steroid receptors.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
CUB domains are not required for OVCH2 function in sperm maturation in the mouse epididymis.Andrology. 2024 Mar;12(3):682-697. doi: 10.1111/andr.13508. Epub 2023 Aug 8. Andrology. 2024. PMID: 37551853 Free PMC article.
-
Knockout of family with sequence similarity 170 member A (Fam170a) causes male subfertility, while Fam170b is dispensable in mice†.Biol Reprod. 2020 Aug 4;103(2):205-222. doi: 10.1093/biolre/ioaa082. Biol Reprod. 2020. PMID: 32588889 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article.
References
-
- Pietras RJ, Szego CM. Endometrial cell calcium and oestrogen action. Nature. 1975;253(5490):357‐359. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

